What We Review
Introduction
The nitrogen cycle is one of the most essential processes supporting life on Earth. It circulates nitrogen through various parts of our planet and ensures that living organisms, including plants and animals, can access this necessary element. Although about 78% of the atmosphere consists of nitrogen N_2, most organisms cannot use it in its atmospheric form. Therefore, nitrogen cycle steps transform atmospheric nitrogen into biologically available compounds such as ammonia and nitrates.
Moreover, the nitrogen cycle highlights the complex interactions between soil, living organisms, and the atmosphere. When all the nitrogen cycle steps flow correctly, healthy ecosystems can thrive. However, disruptions caused by human activities can create imbalances, leading to environmental challenges. Understanding these steps and their significance is crucial for anyone studying AP® Environmental Science.
What Is the Nitrogen Cycle?
The nitrogen cycle involves the continuous movement of nitrogen atoms and molecules among the atmosphere, soil, water, and living organisms. Nitrogen is integral to key biological molecules including proteins and DNA. Consequently, every living cell relies on nitrogen-containing compounds for survival.
However, these compounds must be converted into a usable form before plants and animals can access them. This is why the nitrogen cycle steps are so vital. Most of the time, nitrogen does not remain in any single reservoir for very long. Instead, it transitions through this set of processes—nitrogen fixation, nitrification, assimilation, ammonification, and denitrification—that work together to keep the cycle in balance.
Step-by-Step Breakdown of the Nitrogen Cycle Steps
1. Nitrogen Fixation
Nitrogen fixation begins the nitrogen cycle by converting atmospheric N_2 into ammonia NH_3. Although nitrogen makes up a large part of the air, few organisms can use N_2 directly. Certain bacteria, especially those in association with legumes (such as peas, beans, and clover), have the ability to fix nitrogen. These microbes possess specialized enzymes that break the strong triple bonds in N_2, transforming it into ammonia that plants can use.
Example of Nitrogen Fixation:
- Step 1: Atmospheric N_2 enters the soil.
- Step 2: Nitrogen-fixing bacteria convert N_2 into ammonia NH_3.
- Step 3: Ammonia is taken up by plants to support their growth.
Therefore, nitrogen fixation plays a critical role in supplying new, usable nitrogen to ecosystem food webs.
2. Nitrification
After nitrogen has been fixed into ammonia, the process of nitrification converts ammonia NH_3 into nitrites NO_2^- and then into nitrates NO_3^-. Nitrifying bacteria, which are distinct from nitrogen-fixing bacteria, are responsible for each step in this two-phase conversion. First, certain bacteria oxidize ammonia into nitrites. Next, different species of bacteria oxidize nitrites into nitrates.
Nitrates represent the most common form of nitrogen absorbed by plants. Therefore, the nitrification process fuels plant growth, enabling producers to form proteins and other essential molecules within their tissues.
Example of Nitrification:
- Step 1: Ammonia NH_3 in the soil.
- Step 2: Nitrifying bacteria convert NH_3 into nitrites NO_2^-.
- Step 3: Other bacteria turn nitrites into nitrates NO_3^-, readily absorbed by plants.
3. Assimilation
Assimilation occurs when plants and animals incorporate nitrogen from these inorganic forms into their bodies. Plants primarily take up nitrates NO_3^- from the soil through their roots. Once inside the plant, nitrates are fashioned into amino acids—molecules that serve as building blocks for proteins.
Meanwhile, animals obtain nitrogen by consuming plant tissues (or other animals that have eaten plants). Proteins in a plant’s leaves or seeds ultimately become part of animal tissues like muscle and organs. As a result, nitrogen flows from producers to consumers, fueling growth and vital biological functions.
Example of Assimilation:
- Step 1: Plants absorb nitrates NO_3^- from the soil.
- Step 2: Nitrates facilitate amino acid production within plant tissues.
- Step 3: Animals eat plants, gaining the nitrogen needed for protein synthesis.
4. Ammonification (Decomposition)
Living organisms eventually die or create waste products, which contain organic forms of nitrogen. During ammonification, decomposers—such as certain bacteria and fungi—break down organic matter, releasing ammonia back into the environment. This step closes the loop of organic nitrogen usage by returning it to the soil.
In this way, decaying leaves, fallen trees, and the remains of animals restore ammonia to the environment. These molecules can then be recycled and once more become part of the nitrification process, forming nitrates that support new plant growth.
Example of Ammonification:
- Step 1: Decomposers break down dead plant and animal material.
- Step 2: Organic nitrogen is released in the form of ammonia NH_3.
- Step 3: Ammonia can be processed by nitrifying bacteria, continuing the cycle.
5. Denitrification
While the nitrogen cycle moves nitrogen through soil, plants, and living organisms, it also must return nitrogen to the atmosphere, thus maintaining balance. Denitrification is the step that converts nitrates NO_3^- back into atmospheric N_2. Within low-oxygen environments, specialized denitrifying bacteria use nitrates in their metabolic processes. As a result, they produce N_2 gas, which re-enters the atmosphere.
This step can be especially common in wetlands or waterlogged conditions, where oxygen levels are low. The return of N_2 to the atmosphere completes the cycle, ensuring that nitrogen remains available for fixation and subsequent reuse in ecosystems.
Example of Denitrification:
- Step 1: Nitrates NO_3^- accumulate in soil or waterlogged zones.
- Step 2: Denitrifying bacteria convert NO_3^- into N_2.
- Step 3: Nitrogen gas re-enters the atmosphere, closing the cycle.
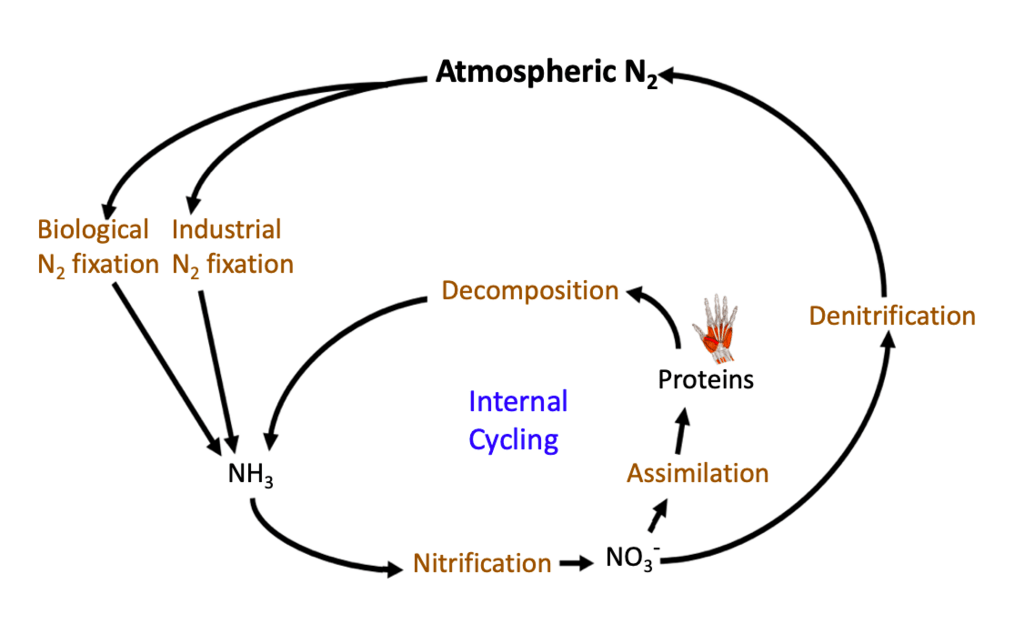
Reservoirs and Short-Term Storage
Although the largest reservoir of nitrogen is the atmosphere, soil and organic matter also serve as significant short-term storage units for nitrogen compounds. The soil, for example, temporarily holds ammonia and nitrates, which plants draw upon for growth. Additionally, organic matter such as dead leaves, wood, and animal remains stores nitrogen in the form of proteins and other compounds. When decomposers break down these materials, nitrogen becomes accessible to plants once again.
Therefore, these reservoirs help regulate the pace at which nitrogen is recycled and limit over-accumulation or shortages in ecosystems. The relatively rapid turnover of nitrogen in soil and organic matter ensures that organisms receive crucial nutrients quickly.
Human Impact on the Nitrogen Cycle
Human activities—particularly the use of synthetic fertilizers—have altered the nitrogen cycle in significant ways. Fertilizers contain high concentrations of nitrogen compounds that boost crop yields. However, excess fertilizer often runs off into waterways, leading to algal blooms. These rapid algae growths can deplete oxygen in aquatic environments, harming fish and other marine life. Furthermore, burning fossil fuels releases nitrogen oxides into the atmosphere, contributing to smog and acid rain formation.
Moreover, large-scale livestock operations produce waste that adds ammonia to soil and water, amplifying nitrogen availability in ways that can exceed natural balance. Therefore, understanding the nitrogen cycle helps illustrate why prudent fertilizer use and responsible industrial practices can reduce harmful environmental impacts. By managing nitrogen applications carefully, societies can protect water quality, aquatic ecosystems, and overall environmental health.
Conclusion
The nitrogen cycle is a fundamental system that supplies organisms with the nitrogen essential for proteins, DNA, and other vital molecules. The nitrogen cycle steps start with nitrogen fixation, when atmospheric N_2 becomes ammonia NH_3. Next, nitrification converts ammonia to nitrates NO_3^-, which plants can readily absorb. During assimilation, plants and animals incorporate nitrogen into their tissues. As organisms die or produce waste, decomposers release ammonia back to the soil through ammonification. Finally, denitrifying bacteria return nitrogen to the atmosphere, completing the cycle. Understanding these steps and their interactions prepares AP® Environmental Science students to analyze ecosystem function and address real-world challenges like agricultural runoff and air pollution.
Key Vocabulary
- Nitrogen Cycle: The series of processes by which nitrogen and its compounds move and transform among the atmosphere, soil, and living organisms.
- Nitrogen Fixation: The conversion of atmospheric nitrogen N_2 into ammonia NH_3, which can be used by plants.
- Nitrification: The bacterial transformation of ammonia NH_3 into nitrites NO_2^- and then nitrates NO_3^-.
- Assimilation: The uptake and use of nitrogen by living organisms to build proteins and other molecules.
- Ammonification: The breakdown of organic nitrogen into ammonia NH_3 by decomposers.
- Denitrification: The conversion of nitrates NO_3^- into atmospheric nitrogen N_2, returning it to the atmosphere.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 1.2 Review
- AP® Environmental Science: 1.3 Review
- AP® Environmental Science: 1.4 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








