What We Review
Introduction
Phosphorus is a critical element that supports life by helping organisms build the molecules necessary for growth, such as DNA and ATP. Unlike the carbon cycle, which moves carbon between air, land, and water, the phosphorus cycle has no significant atmospheric component. As a result, phosphorus does not cycle through the air but, instead, relies on processes like weathering and sedimentation. Consequently, it often becomes a limiting factor in many ecosystems. Understanding how phosphorus moves between its sources and sinks is vital for AP® Environmental Science because it explains why phosphorus sometimes restricts plant growth. Moreover, studying this cycle clarifies how human activities, such as the overuse of fertilizers, influence the health of terrestrial and aquatic ecosystems.
What Is the Phosphorus Cycle?
The phosphorus cycle describes the continuous movement of phosphorus-containing atoms and molecules between various reservoirs in the environment. During this cycle, phosphorus is typically found in the form of phosphate PO_4^{3-}. However, unlike elements such as carbon or nitrogen, phosphorus does not readily enter the atmosphere. Therefore, it is primarily stored in rock, sediments, and living organisms. With no atmospheric shortcut that transfers phosphorus quickly from the oceans back to land, the cycle is relatively slow.
Why No Atmospheric Component?
Phosphorus circulates locally in soils and water sources, but it does not form gaseous compounds that can easily evaporate and return to the atmosphere. Therefore, once phosphorus ends up in the ocean or deep below ground, it may remain there for extended periods until geological upheavals or slow geological weathering processes bring it back to land.
Major Reservoirs of Phosphorus
Phosphorus reservoirs exist where phosphate compounds accumulate in measurable quantities. Rocks and sediments rich in phosphorus-bearing minerals serve as the primary reservoirs on land. These reservoirs include deposits of apatite, a phosphate mineral that forms in both igneous and sedimentary rocks. Because the element does not move through the atmosphere, these geologic reserves constitute the largest storage sites.
- Rock and sediment deposits: The most significant pool of phosphorus, locked away in stable minerals.
- Living organisms: Plants and animals store phosphorus in their tissues, though these biological stores are noticeably smaller.
- Soil and water: Soil particles can bind phosphate PO_4^{3-}, and it dissolves in water in limited concentrations.
In addition, geological uplift and weathering processes eventually expose these phosphorus-rich materials at Earth’s surface. As a result, ecosystems obtain new supplies of phosphorus over very long timescales.
Steps of the Phosphorus Cycle
The cycle progresses in a series of essential steps that move phosphorus from minerals into living organisms and back into the environment. These steps demonstrate how phosphorus flows through food chains.
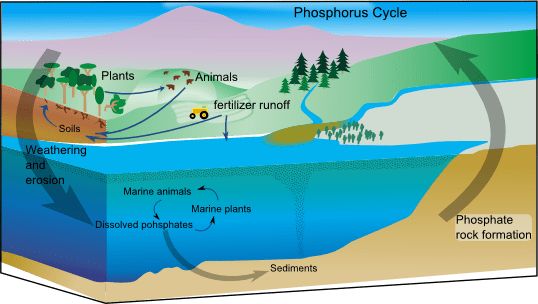
Weathering of Rocks
During weathering, rain, wind, or chemical processes break down rocks containing phosphorus-bearing minerals. Consequently, phosphate PO_4^{3-} substances are released into soils and water.
Example: Over centuries, a rocky hillside exposed to consistent rainfall gradually releases phosphate as rocks crumble.
Step-by-step:
- Rocks break apart due to physical erosion or chemical reactions.
- Phosphorus compounds become small particles carried away by runoff.
- These particles mix with soil and potentially enter water systems, making phosphorus available to plants.
Uptake by Plants
After weathering frees phosphate ions, plants absorb them through their roots. Consequently, phosphorus becomes embedded in tissues, such as stems and leaves. Because phosphate is crucial for processes like photosynthesis and energy transfer, even small amounts can determine how fast plants grow.
Example: A crop field relies on phosphates in the soil to support high yields. When phosphate levels are too low, plant growth slows dramatically.
Step-by-step:
- Soil phosphate ions dissolve in water around plant roots.
- Roots transport the ions into the plant’s vascular system.
- Phosphorus is incorporated into organic compounds like ATP (adenosine triphosphate) and DNA.
Consumption by Animals
When animals feed on plants (or on other animals that have eaten plants), they obtain phosphorus for building their own tissues and carrying out vital functions. As a result, phosphorus moves up the food chain.
Example: In a grassland, zebra herds consume grasses that contain phosphate, transferring this nutrient to their muscles and bones.
Step-by-step:
- Herbivores gain phosphorus from plant tissues.
- Carnivores gain phosphorus by consuming herbivores.
- Phosphorus is used in bones, teeth, and energy molecules in animals.
Decomposition and Recycling
When plants and animals die or excrete waste, decomposers break down organic matter, returning phosphate PO_4^{3-} to the soil or water. Therefore, ecosystems recycle phosphorus in a continuous loop, though some of it may wash away and eventually end up in marine sediments.
Example: In a forest, earthworms, bacteria, and fungi decompose leaf litter on the forest floor, returning phosphate to the soil.
Step-by-step:
- Dead organisms and waste accumulate on the ground or in water.
- Decomposers release phosphate ions from these materials.
- Some phosphate rejoins the soil for plant uptake, while some may travel to larger water bodies.
The Limiting Factor of Phosphorus
A limiting factor in an ecosystem is any element that restricts population growth because it is not available in sufficient quantities. Because phosphorus has no quick atmospheric route for cycling, it tends to be scarce in many landscapes. If plants cannot secure adequate phosphate, they experience stunted growth. Conversely, even a small increase in available phosphorus can produce dramatic surges in plant or algae populations.
Therefore, ecosystems such as freshwater lakes or nutrient-poor soils in tropical regions often rely on very delicate inputs of phosphate. Moreover, the lack of dissolved phosphorus in open waters means that organisms must maximize their use of any small amount that seeps into their environment.
Human Impact on the Phosphorus Cycle
Human activities dramatically alter the flow of phosphorus. Most notably, large-scale fertilizer application increases phosphate levels in agricultural soils. Eventually, this phosphate can run off into rivers and lakes, where it may prompt excessive algae blooms. These blooms deplete oxygen in the water, harming fish and other species in a process called eutrophication.
- Fertilizer use: Farmers rely on phosphate-based fertilizers to boost plant productivity. However, a portion of these nutrients is washed away by rainfall.
- Runoff: Excess phosphorus from farmland, lawns, and even certain detergents enters streams, feeding algae and upsetting normal ecological balances.
- Algal blooms: Explosions of algae growth lead to hypoxic (low-oxygen) or anoxic (no-oxygen) conditions, which reduce biodiversity.
Because of such cascading effects, it becomes crucial to manage fertilizer use responsibly and to prevent the over-enrichment of waterways with phosphorus.
Why Understanding the Phosphorus Cycle Matters
Phosphorus underpins everything from the formation of DNA and cell membranes to the function of ATP, the cell’s “energy currency.” Therefore, even tiny variations in its availability can derail whole food webs. Furthermore, by recognizing how industrial agriculture, waste disposal, and other human practices alter phosphorus flows, scientists and policymakers can develop strategies that maintain stable ecosystems. Healthy phosphorus cycling also promotes sustainable agriculture, protecting future food supplies and preventing damaging algal blooms.
Moreover, studying the phosphorus cycle offers insight into the concept of nutrient availability as a whole. Because phosphorus is often the limiting factor in ecosystems, its dynamics illustrate how even small chemical cycles can have an immense impact on biodiversity, primary productivity, and ecosystem structure.
Key Vocabulary
- Phosphorus Cycle: The continuous movement of phosphorus-containing compounds between rocks, soils, organisms, and sediments, without a significant atmospheric step.
- Limiting Factor: A resource or element that restricts an organism’s growth or population size when it is scarce.
- Weathering: The process that breaks down rocks and minerals at Earth’s surface through physical or chemical means, releasing nutrients.
- Phosphate PO_4^{3-}: The form in which plants typically absorb phosphorus; it is released by weathering or decomposition.
- Eutrophication: The process by which nutrient enrichment (often by phosphorus or nitrogen) in water bodies leads to excessive plant or algae growth, depleting oxygen.
Conclusion
In summary, the phosphorus cycle is a slow but essential pathway that drives plant growth and ecosystem productivity. By tracing how phosphate moves from ancient rocks into living organisms, it becomes clear why phosphorus is frequently the limiting factor in both terrestrial and aquatic environments. These insights help explain the consequences of fertilizer runoff, which accelerates natural phosphorus flows and fuels damaging algal blooms. Ultimately, understanding the phosphorus cycle empowers students and environmental managers to protect ecosystems and sustain agriculture responsibly. Observing how a single nutrient cycles in nature offers a window into broader ecological principles that shape the world.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 1.2 Review
- AP® Environmental Science: 1.3 Review
- AP® Environmental Science: 1.4 Review
- AP® Environmental Science: 1.5 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








