What We Review
Introduction to Air Pollution
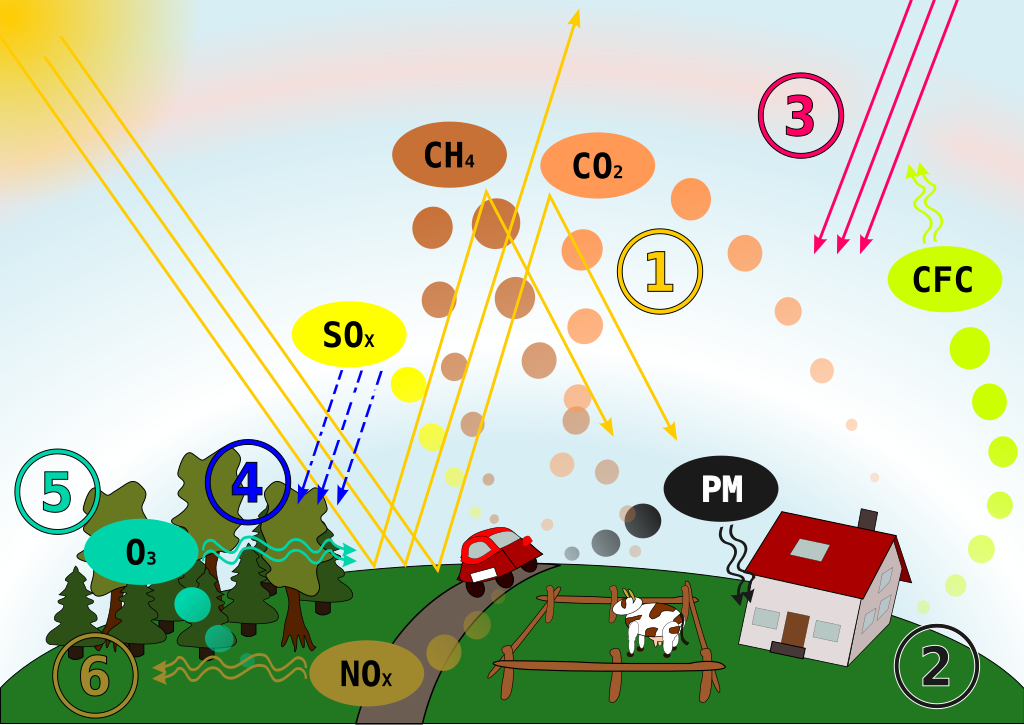
Air pollution refers to the presence of harmful substances in Earth’s atmosphere that can endanger human health, damage the environment, and decrease overall quality of life. In recent decades, understanding this issue has become increasingly important, as various human activities and natural events release gases and particles that contribute to global health and environmental problems. The effects of air pollution stem from multiple sources, including the combustion of fossil fuels, transportation emissions, industrial processes, and even volcanic eruptions.
However, air pollution is not a problem without solutions. Through scientific research and government regulations—most notably the Clean Air Act in the United States—efforts continue to reduce harmful emissions. The following sections explore the major sources, types, and effects of air pollution, as well as regulations established to maintain cleaner air. Ultimately, by understanding how pollutants enter our atmosphere, it becomes possible to manage them more effectively.
Overview of Air Pollution
Definition of Air Pollution
Air pollution occurs when harmful chemicals, particulate matter, or biological materials enter the atmosphere. These substances cause adverse effects on living organisms and the environment. Pollutants include gases like CO_2 (carbon dioxide), SO_2 (sulfur dioxide), and NO_x (nitrogen oxides), along with particulate matter such as dust or soot.
Importance of Understanding Air Pollution
In order to protect both public health and ecosystems, it is vital to study air pollution’s sources and impacts. Many respiratory illnesses—such as asthma—are linked to elevated levels of certain pollutants. Moreover, pollutants like sulfur dioxide can lead to acid rain that harms aquatic environments, while nitrogen oxides contribute to the formation of ground-level ozone and photochemical smog. Therefore, recognizing how pollutants originate and how they move through the atmosphere is essential for effective environmental policies.
Main Sources and Effects
Air pollution results from a variety of factors. Fossil fuel combustion, notably the burning of coal, releases harmful substances that can travel long distances; meanwhile, transportation is a significant source of nitrogen oxides and carbon monoxide. Industrial processes emit toxic chemicals, and natural events like wildfires also inject particulate matter into the air. Over time, these pollutants negatively affect human health, cause environmental degradation, and impose economic burdens through healthcare costs.
Sources of Air Pollution
Fossil Fuel Combustion
Fossil fuel combustion leads to much of today’s air pollution. Coal combustion, in particular, produces several major pollutants, including carbon dioxide, sulfur dioxide, toxic metals (such as mercury), and particulates.
- Explanation of Coal Combustion
- Coal, when burned, generates energy that powers electricity plants. However, coal also contains sulfur and other impurities that are released as gaseous pollutants. In addition, incomplete combustion can produce particulate matter, contributing to smog formation.
- Description of Pollutants Released
- CO_2: A greenhouse gas contributing to climate change.
- SO_2: A gas linked to acid rain formation.
- Toxic metals: Substances like mercury that can pollute waterways.
- Particulates: Fine particles harmful to respiratory health.
- Step-by-Step Example: Pollutants from Coal Combustion
- Coal is fed into a power plant furnace.
- Heat from combustion turns water into steam.
- Steam spins turbines to generate electricity.
- During burning, sulfur in coal converts to SO_2, while carbon forms CO_2
- Particulate matter and toxic metals are released into exhaust gases.
- Emission controls (e.g., scrubbers) partially remove these pollutants before they exit the smokestack.
Transportation Emissions
Vehicles that run on gasoline or diesel release a wide range of pollutants. These include nitrogen oxides, carbon monoxide, volatile organic compounds, and particulate matter. Consequently, heavy traffic in urban areas is often associated with smog events. Over time, tighter regulations on vehicle emissions have helped reduce certain pollutants, though transportation remains a major source of nitrogen oxides that can form ground-level ozone.
Industrial Activities
Many manufacturing processes emit pollutants into the air. Refineries, chemical plants, and cement producers are just a few examples. These industries can release sulfur dioxide, particulate matter, and various toxic substances. Proper industrial design and pollution-control technologies, such as filters and electrostatic precipitators, help reduce harmful emissions.
Natural Sources
Although it might seem surprising, nature also contributes to air pollution. Wildfires, for instance, release large amounts of smoke, particulates, and carbon dioxide. Volcanic eruptions eject sulfur dioxide, ash, and other substances into the atmosphere. Dust storms originating in arid regions carry particulate matter across continents, altering air quality far from their source. These natural events, however, are typically less persistent over time compared to ongoing man-made emissions.
Types of Air Pollutants
Primary Pollutants
Primary pollutants are substances emitted directly into the atmosphere. They include SO_2, NO_x, carbon monoxide, and particulate matter. Because these pollutants are immediately released by a specific source, they can be more readily traced to root causes.
Step-by-Step Example: Primary Pollutant Release
- A refinery burns natural gas for heat.
- As the gas combusts, NO_x is generated.
- NO_x then disperses into the atmosphere.
- This direct emission of NO_x qualifies it as a primary pollutant.
Secondary Pollutants
Secondary pollutants form through chemical reactions among primary pollutants in the atmosphere. One noteworthy example is ozone (O_3) at ground level, created when volatile organic compounds react with nitrogen oxides in sunlight. Photochemical smog in large cities often forms in similar ways, making it more difficult to control than direct emissions.
Example: Ozone Formation
- Sunlight interacts with NO_x and volatile organic compounds.
- Chemical reactions lead to the creation of O_3.
- Ground-level ozone builds up in warm, sunny areas with significant traffic and industrial activities.
Effects of Air Pollution
Human Health
Air pollution harms human health in multiple ways. Short-term exposure can lead to irritation of the eyes, nose, and throat, while long-term exposure is associated with respiratory diseases such as bronchitis and emphysema. Consequently, populations living near industrial hubs or major highways often face higher risks of asthma attacks or cardiovascular problems.
The Environment
Air pollution disrupts ecosystems and forest habitats. An example is acid rain, which happens when sulfur dioxide and nitrogen oxides interact with water vapor, forming acidic compounds. Acid rain damages trees and acidifies lakes, harming aquatic life. Furthermore, excessive ozone can damage plant tissues, stunting growth in crops and reducing agricultural yields.
Example: Acid Rain from Sulfur Dioxide
- SO_2 is released during coal or diesel fuel combustion.
- The gas travels into the upper atmosphere.
- SO_2 reacts with water molecules to form sulfuric acid.
- Acidic droplets fall back to Earth as acid rain, affecting soil and water ecosystems.
The Economy
Air pollution also incurs economic costs. Healthcare systems spend resources treating conditions linked to poor air quality, while industries lose productivity when employees fall ill. In addition, acid rain and smog can affect tourism in regions that rely on clear air and scenic views. Therefore, societies have strong incentives to invest in emissions-reduction technologies and stricter regulations.
Regulation of Air Pollution
Clean Air Act Overview
The Clean Air Act is a comprehensive U.S. law enacted to control air pollution nationwide. It empowers the Environmental Protection Agency (EPA) to establish standards on emissions from vehicles, power plants, and industrial sources. The act includes specific limits on pollutants such as lead, sulfur dioxide, carbon monoxide, nitrogen oxides, and particulate matter. Over the years, government enforcement of these standards has significantly improved air quality in many regions by addressing the effects of air pollution.
Successes and Challenges
Regulation has led to notable successes, including the drastic reduction of lead in the atmosphere after leaded gasoline was phased out. Emission controls, such as catalytic converters in vehicles, have helped limit harmful gases like NO_x and carbon monoxide. However, ongoing challenges remain with greenhouse gas emissions, particulate matter, and ground-level ozone, especially in rapidly developing regions with high industrial growth. Stricter measures and innovative technologies are often necessary to continue improving air quality and addressing new pollution sources.
Conclusion
Air pollution arises from a variety of man-made and natural sources, leading to primary and secondary pollutants that affect human health, ecosystems, and economies. The effects of air pollution are far-reaching, influencing everything from respiratory illnesses to environmental degradation. Nevertheless, steps taken under the Clean Air Act and similar policies highlight the potential for progress when science, regulation, and technology work together. Staying informed about personal and policy-level strategies—such as conserving energy, supporting clean energy initiatives, and adhering to emission standards—can reduce overall air pollution outputs. Continued vigilance and innovation will remain essential for cleaner skies and healthier communities.
Important Vocabulary
- Air Pollution: The presence of harmful substances in the atmosphere.
- Primary Pollutants: Pollutants that are directly emitted into the atmosphere from a source.
- Secondary Pollutants: Pollutants that form in the atmosphere through chemical reactions among other substances.
- Acid Rain: Rain that has been made acidic by pollutants such as sulfur dioxide and nitrogen oxides.
- Clean Air Act: A U.S. law designed to control air pollution on a national level.
- Environmental Protection Agency (EPA): A U.S. federal agency responsible for regulating and enforcing environmental laws.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 6.11 Review
- AP® Environmental Science: 6.12 Review
- AP® Environmental Science: 6.13 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.