What We Review
Introduction
Eutrophication is a critical process that affects the health of many aquatic environments. It frequently occurs in lakes, rivers, and coastal areas, causing an overabundance of nutrients in water systems. These excessive nutrients lead to the rapid growth of algae, subsequently altering the balance of ecosystems. Eutrophication represents one of the most important topics when discussing water pollution and ecosystem dynamics, helping students understand what causes eutrophication and its environmental impacts.
Because healthy water quality is pivotal for aquatic life, understanding eutrophication helps explain why some water bodies experience sudden changes in oxygen levels. This knowledge is also relevant to how human actions, such as fertilizer use, contribute to environmental issues. Therefore, recognizing and mitigating eutrophication remains vital for protecting biodiversity, supporting fisheries, and ensuring safe drinking water supplies.
What Causes Eutrophication?
Definition of Eutrophication
Eutrophication can be described as the enrichment of water bodies with nutrients—primarily nitrogen and phosphorus—that promote plant and algal growth. When these nutrients reach excessive levels, they provoke undesirable shifts in water quality and ecosystem structure. Nutrient overload in aquatic environments creates the perfect conditions for algal blooms.
Explanation of Nutrient Enrichment
Nutrient enrichment happens when external sources of nitrogen and phosphorus enter a water body. These nutrients behave like fertilizers in an agricultural field, nourishing plants and algae. However, an overabundance causes algae to grow uncontrollably, leading to imbalances in aquatic systems. As a result, water clarity often decreases, dissolved oxygen levels drop, and various aquatic organisms struggle to survive.
Common Sources of Nutrients
- Fertilizers: When rain falls on farmlands, it can wash away excess fertilizer and carry it into nearby streams and rivers. This runoff is rich in nitrogen and phosphorus, contributing significantly to eutrophication.
- Detergents: Certain laundry and dishwashing detergents contain phosphates that enrich the water body they enter. Although phosphate‑free detergents are increasingly popular, many regions still experience phosphate pollution from older sources.
Anthropogenic Activities Contributing to Nutrient Overload
While natural phenomena can introduce nutrients into waterways (such as runoff from decaying vegetation), human activity remains the leading driver of eutrophication. For example, applying large amounts of fertilizer to grow crops efficiently can unintentionally supply nearby water bodies with excessive nitrogen and phosphorus. Furthermore, wastewater release from homes, factories, and treatment plants often contains these nutrients. Consequently, these activities become major factors in triggering the eutrophication process.
The Process of Eutrophication
The eutrophication process typically unfolds in several stages. Therefore, examining each step offers insights into how overfertilization leads to reduced dissolved oxygen in aquatic environments.
- Introduction of Nutrients into Water Bodies
- Excess nutrients—often from fertilizers and detergents—flow into streams and rivers. Eventually, these waterways carry nutrients to lakes, reservoirs, or coastal areas.
- Algal Bloom Formation
- With abundant nitrogen and phosphorus, algae multiply rapidly, forming an algal bloom. This bloom can appear as a green or brown layer on the water’s surface, blocking sunlight from reaching deeper.
- Decomposition of Dead Algae
- As algae die, they sink to the bottom. There, bacteria and other decomposers break down the dead algae. The decomposition process consumes large quantities of dissolved oxygen in the water.
- Decrease in Dissolved Oxygen Levels
- The water body becomes hypoxic when microbial activity uses up most of the dissolved oxygen. Dissolved oxygen is critical for fish and other aquatic life, so its reduction threatens the survival of many organisms.
- Consequences for Aquatic Life
- Low oxygen levels commonly lead to fish kills, as most fish need sufficient dissolved oxygen to thrive. Crustaceans and other creatures may also be unable to survive in these conditions. Over time, this chain reaction can transform thriving waterways into dead zones, where few species can persist.
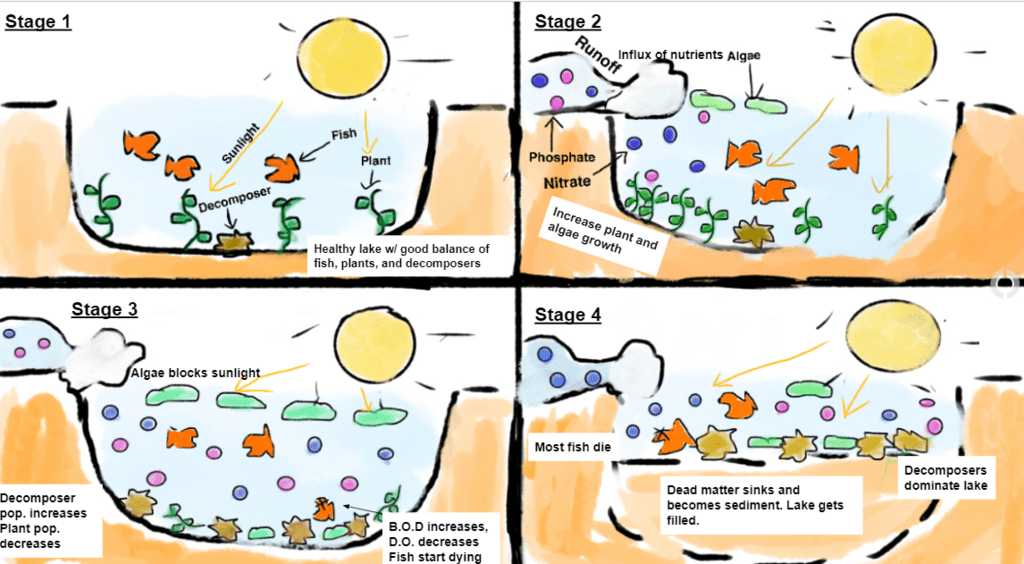
Illustrated Example
Imagine a lake surrounded by farmland where fertilizers are heavily applied to crops. When rain swells the nearby streams, it carries surplus nutrients from the fields into the lake. Next, the lake’s algae receive ample nitrogen and phosphorus, prompting a massive bloom across the surface. Initially, this algal bloom might appear harmless. However, as algae eventually die, oxygen‑consuming microorganisms decompose them. Consequently, dissolved oxygen plummets, and fish often suffocate due to insufficient oxygen. Over time, the lake transitions into a hypoxic or “dead” zone.
The Effects of Eutrophication on Aquatic Ecosystems
A pronounced effect of eutrophication is the formation of hypoxic zones, also called “dead zones.” These zones lack enough dissolved oxygen to sustain most aquatic organisms. Therefore, fish, crustaceans, and other life forms either migrate or die, reducing biodiversity.
Hypoxic Conditions
Eutrophic water bodies become hypoxic because the decomposition process outpaces oxygen re‑supply. In addition, algal blooms limit photosynthesis for other aquatic plants, further decreasing oxygen production. Low oxygen impedes normal metabolic processes in fish, making them vulnerable to suffocation.
Comparing Eutrophic and Oligotrophic Waterways
By contrast, oligotrophic waterways have low nutrient levels, stable algae populations, and high dissolved oxygen. These conditions typically support more diverse communities of fish and aquatic flora. In oligotrophic environments, clear water and consistent oxygen availability promote a healthy balance among algae, plants, and animals.
Case Study: Dead Zones
Dead zones frequently appear where rivers carry large amounts of nutrients into coastal waters. One well‑known example is the Gulf of Mexico’s dead zone, which occurs primarily due to agricultural runoff from the Mississippi River basin. This large influx of fertilizer‑rich water causes major algal blooms, ultimately resulting in oxygen depletion. Consequently, marine life in the affected region significantly declines, impacting local fisheries and ecosystems. Although governments and scientists collaborate on mitigation strategies, the dead zone remains a persistent annual problem.
How to Prevent Eutrophication
Although eutrophication may sound like an unstoppable process, various measures can help reduce nutrient overload. Preventing eutrophication depends on a combination of responsible agricultural practices, improved wastewater treatment, and coordinated community actions.
Best Practices in Agriculture
- Reducing Fertilizer Use: Employing precise fertilizer application methods and using only the necessary amounts can limit nutrient runoff. One effective strategy is to apply fertilizer according to soil test results, ensuring that the land receives only the nutrients it requires.
- Implementing Buffer Zones: Fields planted with strips of grasses or trees next to waterways can filter out excess nutrients before they enter streams and rivers. These buffer zones absorb or trap runoff, reducing the risk of algal blooms downstream.
Wastewater Treatment Improvements
Wastewater from homes and industrial facilities often contains high levels of nutrients, including phosphates from detergents. Upgraded treatment methods can lower the nutrient concentration before water is released back into the environment. For example, technologies that involve biological nutrient removal target native bacteria that consume nitrogen and phosphorus. Consequently, treated water leaves the facility with fewer nutrients, lessening the risk of eutrophication.
Community Involvement and Policies
Successful prevention strategies generally include government regulations and local initiatives. Policymakers may set limits on phosphate content in detergents or require industrial plants to reduce nutrient discharge. Neighborhood organizations can also promote conservation efforts by creating educational programs. For instance, community gardens might adopt low‑fertilizer systems or encourage composting to minimize chemical runoff. Therefore, collaborative efforts unify stakeholders who share the common goal of maintaining healthy waterways.
Conclusion
Eutrophication stands out as a significant concern because it influences both water quality and the survival of aquatic organisms. When waters become overloaded with nutrients, algae proliferate, oxygen is depleted, and habitats transform into hypoxic conditions. As a result, fish kills and biodiversity loss become more frequent.
However, effective management strategies can help prevent or reverse eutrophication. By adopting careful agricultural practices, upgrading wastewater treatment, and supporting community‑based conservation measures, it becomes more feasible to safeguard aquatic environments. Continuing research and public education raise awareness of eutrophication’s impacts, inspiring problem‑solving at multiple levels. Finally, understanding eutrophication is essential for individuals who seek to protect and preserve water ecosystems for future generations.
Key Vocabulary
- Eutrophication: The process by which water bodies become enriched with nutrients, leading to increased plant and algal growth.
- Hypoxic: Referring to water with low dissolved oxygen levels, often harmful to aquatic life.
- Oligotrophic: Water bodies that are low in nutrients and therefore maintain stable algae populations and higher dissolved oxygen.
- Algal bloom: Rapid increase in the population of algae, typically spurred by excessive nutrients.
- Dissolved oxygen: The amount of oxygen in water, crucial for the survival of fish and other aquatic organisms.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 8.2 Review
- AP® Environmental Science: 8.3 Review
- AP® Environmental Science: 8.4 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








