What We Review
Introduction
Ocean acidification is an important topic in AP® Environmental Science because it connects atmospheric conditions with the health of marine ecosystems. This process involves chemical changes in ocean waters that can disrupt marine life, affect biodiversity, and have economic consequences. The effects of ocean acidification are far-reaching, making it essential to understand its global significance and explore ways to mitigate negative impacts. This article examines the causes and effects of ocean acidification, along with potential solutions and action steps.
Ocean acidification refers to the increase in acidity (lower pH) of Earth’s oceans due to the absorption of excess carbon dioxide from the atmosphere. Because the global carbon cycle connects the atmosphere, oceans, and terrestrial systems, changes in one component often ripple throughout the others. In particular, more CO2 in the atmosphere from fossil fuel combustion and deforestation leads to higher CO2 levels in ocean waters. Consequently, marine organisms such as corals, oysters, and clams can struggle to form shells and skeletons. This article will highlight:
- The definition of ocean acidification
- Causes tied to human activities and natural processes
- Effects on marine life, ecosystems, and societies
- Potential solutions and mitigation strategies
What Is Ocean Acidification?
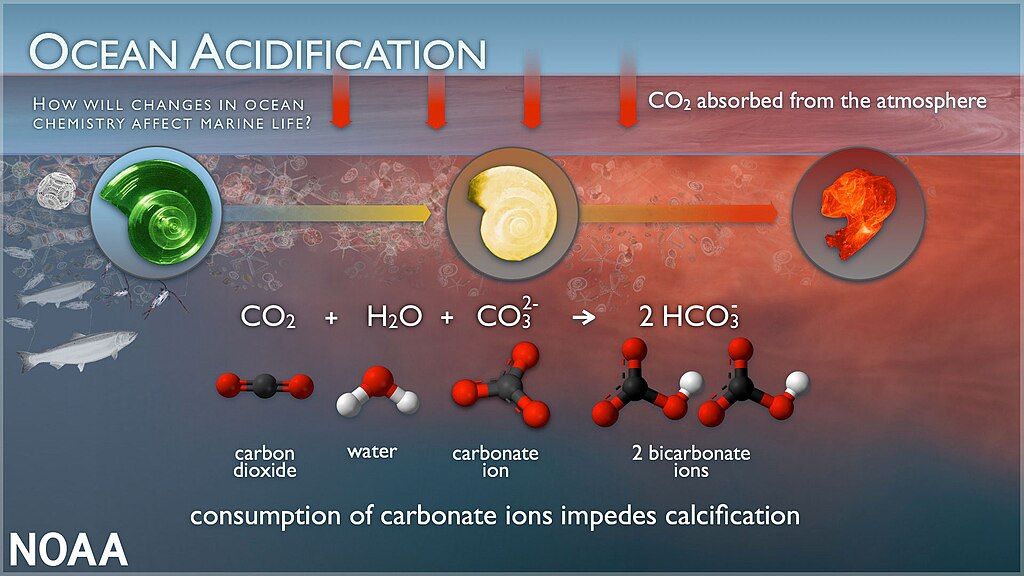
Ocean acidification is a decrease in the ocean’s pH, primarily due to increased \text{CO}_2 concentrations in the atmosphere. The pH scale measures how acidic or basic a substance is. Lower pH values indicate higher acidity, while higher pH values indicate more basic conditions. Because oceans absorb a significant amount of atmospheric \text{CO}_2, the additional dissolved gas undergoes chemical reactions that produce more hydrogen ions. These extra hydrogen ions lead to lower pH values.
The Role of Carbon Dioxide in Ocean Chemistry
When carbon dioxide dissolves in seawater, the reaction can be represented by the following simplified equations:
\text{CO}_2 ,(\text{gas}) \rightarrow \text{CO}_2 ,(\text{aq})\text{CO}_2 ,(\text{aq}) + \text{H}_2\text{O} \rightarrow \text{H}_2\text{CO}_3\text{H}_2\text{CO}_3 \rightarrow \text{H}^+ + \text{HCO}_3^-These steps show that dissolved CO2 becomes carbonic acid (\text{H}_2\text{CO}_3), which then releases hydrogen ions (\text{H}^+) and bicarbonate ions (\text{HCO}_3^-). The presence of \text{H}^+ makes the water more acidic.
Causes of Ocean Acidification
Ocean acidification results from a combination of anthropogenic (human-driven) activities and natural processes. However, human influence has greatly accelerated the rate at which oceans absorb CO2, leading to more pronounced acidity changes than would occur through natural cycling alone.
Anthropogenic Activities
Human activities that increase CO2 levels in the atmosphere include burning fossil fuels, vehicle emissions, and deforestation. These processes release large amounts of carbon dioxide, which circulates globally through the carbon cycle until the oceans take in a significant fraction.
- Burning Fossil Fuels
- Fossil fuels such as coal, oil, and natural gas release CO2 when combusted. Power plants that use these fuels for electricity are major contributors to atmospheric carbon dioxide levels.
- Vehicle Emissions
- Automobiles, trucks, and other vehicles emit CO2 through the combustion of gasoline or diesel. Consequently, heavy traffic and limited public transportation options can intensify CO2 output.
- Deforestation
- Trees naturally store carbon by absorbing CO2 during photosynthesis. When forests are cleared, fewer trees remain to sequester carbon, thus increasing the net CO2 in the atmosphere.
Natural Processes
Natural forces also sustain baseline CO2 levels in the atmosphere. Volcanic eruptions, for example, release CO2 into the environment. Moreover, marine organisms respire and contribute carbon dioxide to the oceans, maintaining a dynamic balance as part of the carbon cycle. However, this balance becomes disrupted when additional CO2 from human activities overloads the system.
Example: Step-by-Step Explanation of CO2 Absorption
- First, carbon dioxide enters the atmosphere through both natural (respiration, volcanoes) and anthropogenic (fossil fuel combustion, deforestation) sources.
- Next, the oceans absorb a portion of this atmospheric CO2 at the air–sea interface.
- Subsequently, once dissolved, carbon dioxide reacts with water to form carbonic acid, decreasing the pH of the ocean.
- Consequently, marine organisms reliant on stable chemical conditions face chemical stress, affecting processes like shell formation.
Effects of Ocean Acidification
Shifts in ocean chemistry have far-reaching impacts on marine life, ecosystems, and human societies. Therefore, understanding effects of ocean acidification is critical for evaluating the significance of rising ocean acidity.
Impact on Marine Life
Corals and shell-producing organisms rely on calcium carbonate (\text{CaCO}_3) for their skeletons and shells. However, the increased presence of hydrogen ions in more acidic waters can limit the availability of carbonate ions (\text{CO}_3^{2-}), a key building block for calcium carbonate structures.
- Coral Reefs: Corals are vital for marine biodiversity because they provide habitat for numerous species. If corals cannot form or maintain their skeletons, reef ecosystems may collapse, endangering fish populations and other marine life that depend on these habitats.
- Shell Formation: Organisms such as oysters, clams, and pteropods (tiny sea snails) depend on carbonate ions to build strong shells. Elevated acidity interferes with shell thickness and strength, making them more vulnerable to predators and environmental pressures.
Ecosystem Changes
When many species in an ecosystem struggle to adapt to more acidic conditions, overall biodiversity may decline. Therefore, species that are more tolerant of acidic waters might outcompete more sensitive organisms, leading to changes in predator-prey relationships and community structure. Furthermore, these shifts can alter nutrient cycles and energy flows within marine environments.
Societal and Economic Effects
Coastal communities often depend on fishing and tourism. Thus, depleted fish stocks and damaged coral reefs can directly affect local economies. Moreover, as coral reefs deteriorate, their ability to protect coastlines from wave action diminishes, increasing the risk of coastal erosion and flood damage.
Example: Step-by-Step Explanation of Coral Reef Damage
- Rising atmospheric CO2 leads to increased absorption of the gas by oceans.
- More dissolved CO2 forms carbonic acid, releasing hydrogen ions.
- Excess hydrogen ions bind to carbonate ions, reducing their availability.
- With fewer carbonate ions, corals cannot effectively build or repair their calcium carbonate structures.
- Gradually, coral reefs weaken and become more susceptible to disease, ultimately risking collapse of the entire reef ecosystem.
An analogy that helps illustrate this process is to imagine trying to assemble a sturdy house with fewer bricks available. If materials are scarce, the walls become weaker and might eventually break down.
Solutions and Mitigation Strategies
Because ocean acidification is tied to elevated CO2 levels, effective solutions primarily involve reducing carbon emissions and promoting sustainable practices. Policies, research, and individual actions all play a role.
Reducing Carbon Footprint
Lowering the reliance on fossil fuels and adopting renewable energy are two crucial paths toward mitigating ocean acidification. By switching to solar, wind, and other low-carbon technologies, communities can shrink total CO2 emissions. Additionally, energy-efficient homes, public transportation, and mindful consumption behaviors can lessen carbon footprints.
Policy Changes
Governments can influence large-scale CO2 emissions by establishing stricter regulations on industries and mandating cleaner fuel standards. Carbon taxes or cap-and-trade systems can also encourage companies to pursue cleaner alternatives. Furthermore, protecting forests through legislation helps maintain the planet’s capacity for carbon sequestration, thereby limiting the amount of CO2 that enters the atmosphere.
Research and Monitoring
Scientists continuously monitor pH levels across different ocean regions to track changes in water chemistry. Ongoing research into ocean acidification mechanisms, coral resilience, and species adaptation can guide future marine conservation strategies. Therefore, supporting scientific investigation is an important component of mitigating the long-term consequences of ocean acidification.
Example: Step-by-Step Guide to Reducing Personal Carbon Footprint
- Evaluate transportation habits: Increasing carpooling or using public transit helps decrease vehicle emissions.
- Conserve energy at home: Turning off lights and electronics when not in use and upgrading to energy‑efficient appliances can reduce electricity consumption.
- Adjust dietary choices: Eating locally grown foods requires less transportation and energy, lowering overall CO2 emissions.
- Support reforestation: Donating to tree-planting initiatives or participating in local plant-a-tree events can help remove CO2 from the atmosphere.
Implementing these simple changes can gradually reduce the global CO2 load, helping to slow ocean acidification.
Conclusion
Ocean acidification is a significant challenge that arises from excessive carbon dioxide levels in the atmosphere. Burning of fossil fuels, vehicle emissions, and deforestation accelerate the process by adding substantial amounts of CO2 that oceans subsequently absorb. This change in ocean chemistry harms marine organisms by hindering shell and reef formation, potentially leading to ecosystem imbalances and economic losses for coastal communities. Nevertheless, efforts to lessen CO2 emissions—through policy changes, individual actions, and research—offer a path toward protecting marine life and safeguarding the essential services oceans provide. Recognizing how interconnected the global carbon cycle truly is can inspire broader support for climate solutions, ultimately helping to preserve ocean health.
Key Vocabulary
- Ocean Acidification: A decrease in the pH of the ocean caused by increased \text{CO}_2 levels, making seawater more acidic.
- pH: A scale measuring acidity or alkalinity; lower values indicate higher acidity, while higher values indicate a more basic solution.
- Carbon Dioxide (CO2): A greenhouse gas produced by burning fossil fuels, vehicle emissions, and deforestation, contributing to ocean acidification.
- Fossil Fuels: Energy sources such as coal, oil, and natural gas that release large amounts of \text{CO}_2 when burned.
- Deforestation: The removal of forests that reduces the number of trees available to absorb \text{CO}_2 from the atmosphere.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 9.4 Review
- AP® Environmental Science: 9.5 Review
- AP® Environmental Science: 9.6 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








