What We Review
Introduction
Acid rain is a critical topic because it illustrates the interaction between atmospheric processes and ecosystems. Students often examine acid rain to understand how human activities can disrupt environmental balance. Exploring acid rain requires grasping what causes acid rain, its formation, environmental consequences, and potential solutions. The following sections break down these concepts in a clear and concise manner.
Acid rain refers to precipitation that has a lower pH than normal rain, making it more acidic. Normal rain has a pH of around 5.6 due to the natural presence of carbon dioxide in the atmosphere. However, acid rain can have a pH of 4.4 or even lower. Understanding acid rain is pivotal for those preparing for the AP® Environmental Science exam because it ties together themes of air pollution, biogeochemical cycles, and community-level impacts. Therefore, an in-depth look at acid rain helps students see the connections among various environmental processes and the role humans play in them.
What Is Acid Rain?
Acid rain is a general term for any form of precipitation—rain, snow, or sleet—that contains acidic components, such as sulfuric or nitric acid. Its defining feature is a pH level below 5.6. Acid rain develops when certain pollutants in the air dissolve into water droplets, forming acidic compounds. Nitrogen oxides (NO_x) and sulfur oxides (SO_x) are the primary contributors to this process.
The Role of pH
The pH scale measures how acidic or basic a substance is. It runs from 0 (most acidic) to 14 (most basic). The midpoint value of 7 is considered neutral. Rainwater is naturally slightly acidic because it absorbs some carbon dioxide from the atmosphere, producing weak carbonic acid. However, human-induced emissions of nitrogen oxides and sulfur oxides lead to much higher levels of acidity, which is what distinguishes acid rain from normal precipitation.
Example: Normal Rain vs. Acid Rain
- Normal rain typically has a pH around 5.6.
- Acid rain can have a pH as low as 4.4 or even lower in regions with heavy industrial emissions.
This difference may seem small numerically, but each unit change in pH represents a tenfold change in acidity. Consequently, a drop from 5.6 to 4.6 means the rain is ten times more acidic.
Causes of Acid Rain
Acid rain stems from both natural and anthropogenic sources. Most discussions emphasize anthropogenic sources, because these are the primary contributors and are also the most easily controlled through regulatory practices.
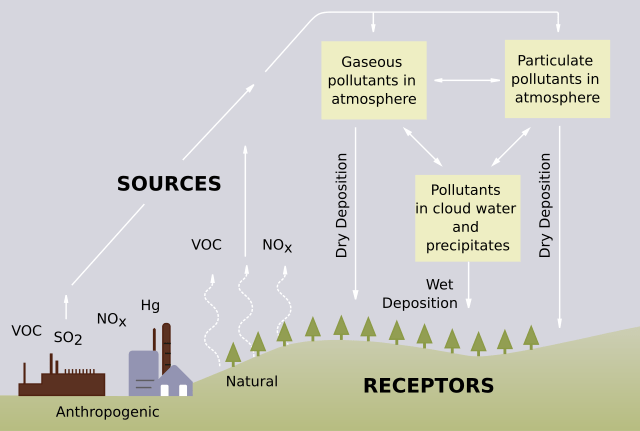
Natural Sources
Certain events in nature can lead to the release of nitrogen and sulfur compounds, which then become part of the atmosphere:
- Volcanic Eruptions: Volcanoes can spew SO_2 (sulfur dioxide) into the air.
- Lightning: Lightning strikes can help produce NO_x (nitrogen oxides) at high altitudes.
Although these natural sources exist, their contribution to overall acid rain problems is relatively small compared to human-made sources, which are central to understanding what causes acid rain.
Anthropogenic Sources
Human activities account for the largest share of nitrogen oxides and sulfur oxides in the atmosphere. Coal-burning power plants are a significant source of sulfur oxides, especially SO_2. Meanwhile, other industrial processes also add to the sulfur oxide load. Nitrogen oxides are largely emitted by trucks, cars, and other vehicles with internal combustion engines. When these gases enter the atmosphere, they can remain there until they react with water vapor to form acids.
The Role of Motor Vehicles
Motor vehicles produce large amounts of NO_x due to high-temperature combustion in engines. Because vehicles are everywhere, their combined emissions significantly contribute to acid rain. The more traffic and fossil fuel consumption a region has, the greater the emissions of nitrogen oxides. Consequently, urban areas often experience higher levels of acid rain precursors.
Example: Emissions from a Coal-Burning Plant
- A coal-burning power plant emits sulfur dioxide (SO_2) and nitrogen oxides (NO_x).
- These gases travel through the air and react with oxygen, forming SO_3 and various nitrogen compounds.
- The gases then mix with atmospheric water vapor.
- Chemical reactions produce strong acids such as sulfuric acid (H_2SO_4) and nitric acid (HNO_3).
- When precipitation occurs, these acidic compounds return to Earth’s surface as acid rain.
Environmental Effects of Acid Rain
Acid rain can harm diverse ecosystems and human-made structures. Its effects become most noticeable in regions that lack natural buffering systems. Transition words such as “consequently” and “furthermore” help clarify these impacts.
Impact on Soils
Acid rain increases soil acidity, which can harm plants by leaching essential nutrients such as calcium and magnesium from the soil. When these nutrients are flushed out, plant growth may decline. Acidification can also mobilize toxic metals like aluminum, further stressing or killing vegetation.
Effects on Water Bodies
Lakes, streams, and rivers can exhibit dramatic changes in water chemistry under acid rain conditions. As pH drops, aquatic species sensitive to acidic environments, such as certain fish and insects, may die or fail to reproduce. Therefore, biodiversity can plummet in ecosystems constantly exposed to acid rain.
Corrosion of Human-Made Structures
Acidic precipitation can corrode buildings, monuments, and infrastructure. For example, marble and limestone structures are vulnerable because these materials react chemically with acids, leading to discoloration and structural weakening. Consequently, important cultural artifacts and architectural heritage can suffer long-term damage.
Example: Soil Acidification in Action
- Acidic precipitation falls on forest soil.
- Essential nutrients like calcium become less available to tree roots.
- Toxic metals, including aluminum, become more soluble and damage root systems.
- Tree growth slows, and overall forest health declines.
- Eventually, ecosystem productivity decreases, affecting biodiversity and carbon storage.
Regional Differences in Impact
Not all regions experience the environmental effects of acid rain equally. Several geological and biological factors can influence how severely an area is affected. Some regions have natural neutralizing agents, while others do not.
- Soils with high buffering capacity: Areas rich in limestone (CaCO_3) tend to resist acidification because limestone neutralizes acids by reacting with them.
- Soils with low buffering capacity: Regions lacking substantial limestone deposits are more sensitive. Their lakes or streams can rapidly become acidic because no natural neutralization occurs.
- Wind patterns: Communities downwind from coal-burning facilities receive a large share of acid deposition, even if their local emissions are low.
Example: Limestone vs. Non-Limestone Regions
- Region A: Soils rich in limestone. Rain that falls on these soils becomes neutralized, so pH levels do not drop dramatically. Plants and aquatic life show fewer negative effects.
- Region B: Soils without limestone. Precipitation acidity remains high, causing more rapid soil and water acidification. Ecosystems in this area face greater stress from acid deposition.
Mitigation and Solutions
Although acid rain poses serious environmental challenges, multiple strategies exist to reduce its occurrence and mitigate its effects. Cleaner energy sources, emission controls, and regional regulations play essential roles in curbing acid deposition.
- Switching to renewable energy: Solar, wind, or hydroelectric sources emit fewer pollutants, reducing NO_x and SO_x levels.
- Installing scrubbers in power plants: Scrubbers remove sulfur oxides from power plant exhaust, thereby lowering emissions.
- Enforcing environmental regulations: Laws like the Clean Air Act in the United States set allowable limits for sulfur dioxide and nitrogen oxide emissions.
- Encouraging community awareness: Involving residents in educational campaigns fosters environmentally responsible behavior, such as carpooling or using public transport.
Example: Community Initiative to Reduce Acid Rain
- Local government organizes a workshop explaining acid rain formation and its harmful effects.
- Community members decide to promote car-sharing programs to decrease vehicle emissions.
- Area schools adopt green energy solutions, like installing solar panels, thereby reducing reliance on fossil fuels.
- Monitoring stations track local air quality and rainfall pH levels over time.
- Community members compare data daily to see if their collective efforts are making a positive difference in acid rain reduction.
Conclusion
Acid rain remains a substantial concern in environmental science because it connects atmospheric chemistry and ecosystem health. By examining how nitrogen oxides and sulfur oxides form acids in the atmosphere, students gain insights into what causes acid rain and the link between industrial activities and environmental stressors. Learning about acid rain equips future decision‑makers to enact policies and lifestyle changes that protect soils, water bodies, and structures. Ultimately, understanding acid rain encourages a more informed approach to environmental protection and urges ongoing innovation in cleaner energy and community strategies.
Key Vocabulary
- Acid Rain: Precipitation with a pH lower than 5.6, formed when water droplets mix with nitrogen and sulfur oxides in the atmosphere.
- Nitrogen Oxides: A group of gases produced mainly by motor vehicles and coal-burning plants, which contribute to acid rain.
- Sulfur Oxides: Pollutant gases released by fossil fuel combustion, especially from coal-burning power plants, that form sulfuric acid in the atmosphere.
- pH: A measure of acidity or alkalinity, ranging from 0 to 14, where lower values are more acidic and higher values are more basic.
- Limestone: A type of sedimentary rock that can neutralize acids, thereby helping to reduce the impact of acid rain on soils and water bodies.
- Anthropogenic: Originating from human activities or influences, such as industrial emissions and motor vehicle exhaust.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 7.4 Review
- AP® Environmental Science: 7.5 Review
- AP® Environmental Science: 7.6 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








