What We Review
Introduction
Carbon plays an essential role in supporting life on Earth. It forms the backbone of many biological molecules, including proteins and carbohydrates. As part of the natural environment, carbon influences processes such as the formation of fossil fuels and the regulation of atmospheric gases. Therefore, understanding the carbon cycle steps and how carbon moves through different systems is crucial. In AP® Environmental Science, the carbon cycle highlights the constant flow of carbon atoms between living organisms (biotic components) and nonliving elements (abiotic components), such as the atmosphere and the oceans.
The carbon cycle steps also connects to other environmental topics, including the global climate system, population growth models, and renewable energy choices. Even a slight change in the balance of carbon fluxes can affect temperatures worldwide, as seen in recent discussions of climate change. Hence, studying the carbon cycle helps learners grasp some of the most pressing environmental issues facing the planet today.
What Is the Carbon Cycle?
The carbon cycle refers to the continuous movement of carbon atoms between sources and sinks in the biosphere, geosphere, atmosphere, and hydrosphere. Carbon is constantly recycled and reused. Some reservoirs—such as rock formations in Earth’s crust—store carbon compounds for millions of years. Others—such as living organisms—store carbon for much shorter periods.
These movements occur through a series of processes, including photosynthesis, respiration, decomposition, and fossil fuel combustion. Because carbon is fundamental to life, these processes impact organisms, ecosystems, and even climate patterns. In the following sections, each of the carbon cycle steps is outlined in detail, followed by a description of the major carbon reservoirs.
The Carbon Cycle Steps
1. Photosynthesis
It represents the process through which green plants, algae, and certain bacteria convert carbon dioxide CO_2 and water H_2O into glucose C_6H_{12}O_6 and oxygen O_2.
- Step-by-step process:
- Pigments in plant leaves, typically chlorophyll, absorb light energy from the Sun.
- The absorbed energy splits water molecules, releasing O_2.
- The plant takes in CO_2 from the atmosphere.
- The energy fixes CO_2 into glucose, C_6H_{12}O_6, which plants use to grow and function.
Photosynthesis is significant because it removes CO_2 from the atmosphere, storing carbon in plant tissues. Consequently, this process helps regulate atmospheric carbon levels.
2. Respiration
Respiration is almost the reverse of photosynthesis. Organisms (including plants) break down glucose in the presence of oxygen to release energy, water, and CO_2. The general equation for respiration is:
C_6H_{12}O_6 + O_2 \rightarrow CO_2 + H_2O- Step-by-step process:
- Cells consume glucose, either obtained by eating plants (or organisms that ate plants) or by breaking down stored sugars.
- When glucose reacts with oxygen, it releases energy that cells use for growth and repair.
- Carbon dioxide is released back into the environment, returning carbon to the atmosphere.
Therefore, photosynthesis and respiration form a cycle that moves carbon in a loop between producers and consumers.
3. Decomposition
Decomposition involves the breakdown of dead organisms and waste products by decomposers (bacteria, fungi, and certain insects). During this process, decomposers respire. They consume organic matter, converting carbon-rich substances into simpler compounds.
- Step-by-step process:
- Decomposers identify and break down dead matter, recycling nutrients in the ecosystem.
- As they respire, CO_2 is released into the atmosphere or dissolves in the soil and water.
- The various byproducts of decomposition enrich soils, making them more fertile.
Decomposition ensures that carbon does not remain “locked” in dead tissue. Instead, it is steadily returned to the environment, where it can again enter the cycle.
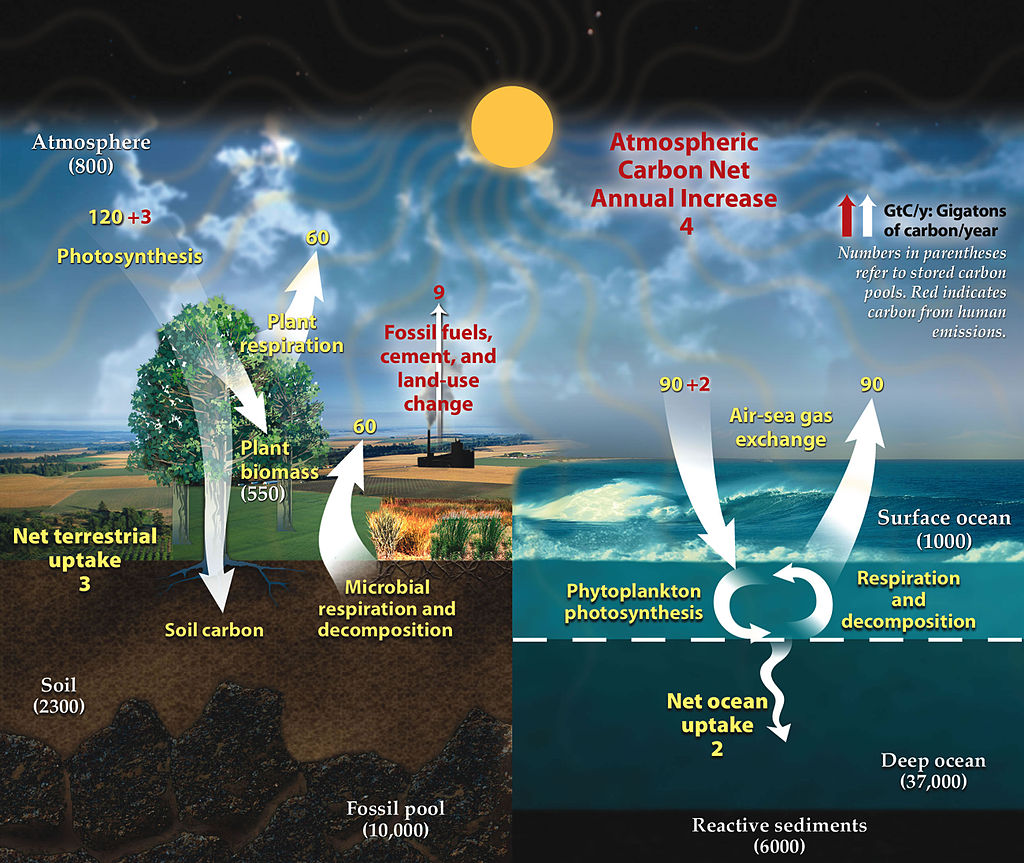
4. Fossil Fuel Formation and Combustion
Over millions of years, the remains of ancient plants and animals can be buried under sediment and rock, where intense heat and pressure transform them into fossil fuels such as coal, oil, and natural gas.
- Formation:
- Plant or animal remains accumulate in sediment.
- Layers of sediment build pressure and temperature over millions of years.
- Carbon-rich fossil fuels (coal, oil, or gas) form deep beneath the surface.
- Combustion:
- When fossil fuels are extracted and burned, the stored carbon rapidly converts into CO_2.
- This CO_2 increases atmospheric carbon levels, contributing to global climate change.
- The rapid release of carbon from ancient reservoirs can upset the natural balance of the carbon cycle.
Human reliance on fossil fuel combustion is one of the key drivers of modern climate shifts, which is why renewable energy is an appealing alternative.
Reservoirs of Carbon
Carbon reservoirs are places where carbon compounds are stored for varying lengths of time. Some reservoirs hold carbon for long periods, whereas others store it briefly.
- Short-term reservoirs include the atmosphere and living organisms. Carbon can circulate here on timescales of days to years.
- Medium-term reservoirs include the ocean’s surface waters, where CO_2 dissolves. Some marine organisms incorporate carbon into shells, creating calcium carbonate structures that may later become sedimentary rock.
- Long-term reservoirs exist in fossil fuels and deep geological formations. Here, carbon may remain locked away for millions of years until geological or human activities release it.
Therefore, the duration of carbon storage varies widely, influencing how quickly or slowly it recycles through the environment.
The Impact of Human Activity on the Carbon Cycle
Human actions immediately affect how carbon moves through its cycle. The burning of fossil fuels rapidly transfers ancient carbon to the atmosphere, raising its CO_2 concentration. In tandem, deforestation removes critical carbon sinks, because fewer trees remain to capture CO_2 through photosynthesis. Consequently, the depleted forests store less carbon, and the excess CO_2 exacerbates greenhouse effects.
Climate change is closely tied to these shifts in carbon balance. As CO_2 and other greenhouse gases accumulate, Earth’s surface temperatures tend to rise. This phenomenon may lead to melting polar ice, sea-level rise, and further disruptions to ecosystems and weather patterns. Understanding these relationships is essential for AP® Environmental Science students who study the link between human society and environmental processes.
Interactive Example: Following a Carbon Atom Through the Cycle
Consider a hypothetical carbon atom, “C,” beginning its journey in the atmosphere as part of a CO_2 molecule. This molecule is absorbed by a leaf during photosynthesis. Next, plants use C to form glucose molecules. An insect feeds on this plant, digesting the glucose during cellular respiration. This reaction releases some carbon back into the atmosphere as CO_2. The insect eventually dies, and decomposers break down its body, again releasing more CO_2.
However, imagine some carbon-17 from a massive ancient forest buried under sediment in a different timeline. Over millions of years, it becomes coal. When that coal is burned in a power plant, the stored carbon is released in the form of CO_2. Therefore, a single carbon atom can take multiple paths but will inevitably rejoin the atmosphere or another reservoir in due time.
Importance of Understanding the Carbon Cycle
A solid grasp of the carbon cycle supports insight into far-reaching environmental issues. It clarifies why carbon emissions drove the Industrial Revolution and why they continue affecting global temperatures. This knowledge also allows students to evaluate mitigation strategies, such as transitioning to renewable energy, reforestation, or carbon capture technologies.
AP® Environmental Science students apply these lessons when examining pollution control measures, ecological footprints, and resource management. Therefore, studying the carbon cycle lays the foundation for a deeper awareness of human impacts and nature’s capacity to respond.
Key Vocabulary
- Carbon Cycle: The continuous movement of carbon atoms among living organisms, the atmosphere, oceans, and Earth’s crust.
- Photosynthesis: The process by which producers convert CO_2 and H_2O into glucose (C_6H_{12}O_6) and O_2 using sunlight.
- Cellular Respiration: The metabolic process in which glucose is broken down with oxygen to release energy, CO_2, and H_2O.
- Carbon Reservoir: A storage area for carbon, ranging from short-term (living organisms) to long-term (fossil fuels).
- Fossil Fuel Combustion: The burning of ancient organic matter, releasing stored carbon into the atmosphere as CO_2.
Conclusion
The carbon cycle touches nearly every aspect of environmental science, from microscopic processes within cells to massive shifts in global climate patterns. It defines how carbon moves among different reservoirs and facilities, affecting both natural ecosystems and human society. Recognizing the links within this cycle helps clarify current challenges, such as escalating greenhouse gas emissions and rapid deforestation.
For AP® Environmental Science, mastering the carbon cycle steps ensures a deeper appreciation of life’s interconnectedness. This knowledge also reveals avenues for solutions, encouraging exploration of energy systems, land use policies, and innovative technologies. By maintaining balance in the carbon cycle, present and future generations can protect the planet’s climate and biodiversity.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








