What We Review
Introduction
Fuels power vehicles, heat homes, and illuminate cities. They also play a vital role in the global economy. It is critical to understand different fuel types, how they are produced, and their environmental effects. It helps in making informed decisions about resource management and sustainability. This article explores both common and emerging fuel sources, highlighting their uses and the environmental considerations they carry.
Fuel sources often fall into two primary categories: renewable and non-renewable fuel types. However, the details can become more nuanced when discussing specific fuels, such as wood, peat, coal, natural gas, and crude oil. Many of these fuels are considered non-renewable since they take millions of years to form. Yet, wood is often classified differently due to how quickly it can be replenished if managed responsibly.
Renewable vs. Non-Renewable Fuels
Renewable fuels derive from resources that can be replenished within a human timescale. Wood from sustainably managed forests is considered one such renewable resource. In contrast, non-renewable fuels, such as coal and crude oil, form slowly through geological processes. These take far longer to regenerate than the rate at which humans consume them. Therefore, it is important to understand which fuels can be obtained repeatedly without depleting resources.
Renewable fuels typically have a lower environmental impact because they contribute less to long-term carbon dioxide buildup in the atmosphere. However, they still produce emissions when burned. Non-renewable fuels, on the other hand, often create significant emissions that can lead to climate change concerns and other environmental effects. Consequently, choosing the right combination of renewable and non-renewable fuels can be a balancing act between practicality, cost, and ecological impact.
Example – Tracking Carbon Emissions:
- Imagine two communities, each using a different fuel type for heating.
- Community A uses sustainably harvested wood, ensuring new trees are planted after each harvest.
- Community B uses coal, a non-renewable resource formed over millions of years.
- Over the same period, Community B releases more carbon dioxide without the immediate possibility of “re-sequestering” carbon naturally.
- This example underscores how renewable fuels can help offset carbon emissions when managed properly.
Common Fuel Types and Their Uses
Wood
Wood is an accessible fuel source in many regions. It is often consumed as firewood or charcoal. In many developing nations, wood remains a primary cooking and heating fuel because it is relatively easy to gather. Carefully managed forests can renew wood supplies. However, unsustainable harvesting can lead to deforestation.
Example – Comparing Firewood and Charcoal Step by Step:
- When wood is burned directly as firewood, it releases heat but also produces smoke and particulates.
- Charcoal results from heating wood in a low-oxygen environment so that water and volatile compounds evaporate, leaving mostly carbon.
- Because charcoal is denser in carbon, it burns more efficiently and with less smoke.
- Thus, users often prefer charcoal in settings where cleaner combustion is crucial, but the production process requires additional energy upfront.
Peat
Peat consists of partially decomposed organic matter found in wetlands, such as bogs and moors. Over time, plant material accumulates in waterlogged conditions that prevent full decay. This organic material can be dried and burned as a fuel, producing significant heat.
Formation and Extraction Example:
- Plant debris accumulates in waterlogged soils, forming layers of organic matter.
- Because these soils lack enough oxygen, decomposition is incomplete, resulting in peat.
- Workers drain or cut portions of peat from the peatland, then leave it to dry.
- Once dry, peat can be ignited for heating because of its high carbon content.
- However, large-scale peat extraction can harm wetland ecosystems, leading to habitat loss and increased carbon dioxide release.
Coal
Coal is a hard black or brownish rock composed mostly of carbon. It forms from plant remains buried under geological layers for millions of years. Coal is typically classified into three types, each varying in carbon content and energy potential:
- Lignite (lowest carbon content)
- Bituminous (medium carbon content)
- Anthracite (highest carbon content)
Depth of Burial Example:
- As plant material gets buried deeper beneath layers of sediment, pressure and temperature rise.
- This continuous heat and pressure transform the organic material first into lignite.
- With deeper burial, the coal matures into bituminous coal.
- Eventually, extreme pressure and temperature can produce anthracite, the highest-grade coal with the highest carbon content.
- Each step in this process takes thousands or millions of years, making coal a non-renewable resource.
Natural Gas
Natural gas mainly consists of methane (\text{CH}_4). Because it burns more cleanly than coal or oil, it is often considered the cleanest fossil fuel. It is widely used for heating and cooking, and it can also be used in power plants to generate electricity. Due to its relatively low carbon dioxide emissions compared to coal, many energy policies favor shifting toward natural gas to reduce greenhouse gas outputs.
Heating and Cooking Example:
- A typical home might have a natural gas furnace that ignites methane to release heat.
- The burnt methane produces carbon dioxide and water vapor.
- Kitchens can use natural gas stoves for consistent and controllable heat.
- Thus, many households prefer natural gas due to its lower emissions compared to other fossil fuels.
- However, leaks of unburned methane can still increase greenhouse gas levels because methane is a potent greenhouse gas.
Crude Oil
Crude oil is a complex mixture of hydrocarbons formed from ancient plankton and algae remains. Over millions of years, pressure and heat transform this biomass into liquid petroleum trapped in rock formations. Sometimes crude oil is extracted from tar sands, which are mixtures of sand, clay, water, and bitumen. Refining processes convert crude oil into various products such as gasoline, diesel, and other petrochemicals.
Refining Process Example:
- Crude oil is transported to a refinery, where it is heated in a distillation tower.
- Lighter hydrocarbons vaporize at lower temperatures, rising to higher levels in the tower.
- Gasoline components collect near the top, where it is cooler.
- Heavier residues remain lower in the tower.
- This step-by-step separation allows refineries to produce specialized fuels for motor vehicles, aviation, and other uses.
Fossil Fuels and Their Specialized Uses
Fossil fuels include coal, natural gas, and crude oil. Each has unique physical and chemical properties. Specialized uses often rely on the particular characteristics of each fuel type. For instance, gasoline produced from crude oil is well-suited to internal combustion engines in cars because it ignites easily and releases high energy per unit volume. Coal is favored in certain industrial processes, such as steel manufacturing, due to its high heat output at relatively low cost.
Example – Transforming Fossil Fuels for Motor Vehicles:
- Crude oil is refined to isolate hydrocarbons that vaporize at specific temperature ranges.
- Blended fuels, such as gasoline, contain additives to enhance their performance in engines.
- Engines burn the fuel, creating gases that drive pistons.
- Although efficient, this process generates carbon dioxide, prompting initiatives to develop alternative and renewable fuels.
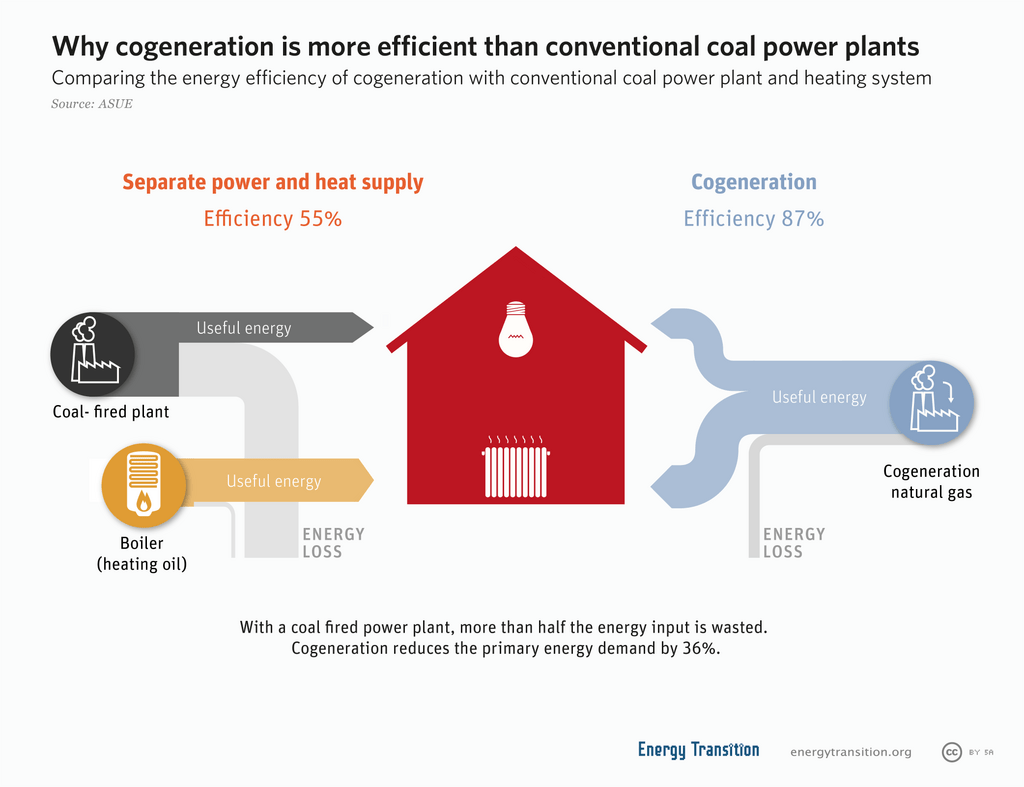
Cogeneration: Maximizing Fuel Efficiency
Cogeneration, sometimes referred to as combined heat and power, involves using one fuel source to produce both electricity and usable heat at the same time. In typical power plants, much of the heat generated during electricity production is wasted. Cogeneration captures this waste heat and uses it to warm buildings or power industrial processes.
Basic Cogeneration Plant Operation:
- A fuel, such as natural gas, is burned in a turbine to generate electricity.
- The combustion process releases heat, which is often vented in traditional plants.
- In a cogeneration facility, captured heat is used to produce steam.
- This steam can be piped into buildings for heating.
- As a result, overall efficiency ramps up, reducing the quantity of fuel consumed and cutting emissions.
The Future of Fuel: Trends and Considerations
As renewable energy technologies advance, many nations are switching to cleaner fuel options. Solar panels, wind turbines, and other sustainable methods offer paths to reduce dependence on non-renewable fuels. However, transitioning entirely to renewable energy does not happen overnight. Infrastructure changes, policy shifts, and economic factors all influence how quickly a region adopts sustainable fuels.
Therefore, a balanced energy approach often includes natural gas as a bridge year while renewable energy capacity is built. Although renewable fuels such as wood or biomass may work for some communities, these options still emit carbon when burned. Thus, the responsibility lies in managing resources so that emissions can be offset. Over time, increased energy efficiency, better technology, and ongoing research will guide the future of fuel choices worldwide.
Conclusion
Fuel types range from ancient peat deposits to modern forms of biomass. Each option carries unique benefits and drawbacks. Non-renewable fuels like coal and oil offer high energy density, yet they contribute heavily to greenhouse gas emissions. Renewable fuels, including sustainably harvested wood, can potentially reduce environmental impact, but only if managed carefully.
When preparing for the AP® Environmental Science exam, it helps to grasp how different fuels form, why they are classified as renewable or non-renewable, and what specific uses they serve. Understanding these fuel fundamentals empowers individuals and communities to make cost-effective, eco-friendly decisions, reflecting a growing emphasis on sustainability.
Key Vocabulary
- Wood: Fuel derived from trees, commonly used as firewood or charcoal.
- Peat: Partially decomposed organic matter found in waterlogged soils that can be dried and burned.
- Lignite: A type of coal with the lowest carbon content, typically formed at shallow burial depths.
- Bitumen: A thick, sticky form of petroleum found in tar sands that can be refined into fuels.
- Cogeneration: A system where one fuel source produces both electricity and usable heat, boosting efficiency.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 5.17 Review
- AP® Environmental Science: 6.1 Review
- AP® Environmental Science: 6.2 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.