What We Review
Introduction
Ozone depletion remains a significant challenge in environmental science. It directly affects the health of ecosystems, as a damaged ozone layer allows more harmful ultraviolet (UV) radiation to reach the Earth’s surface. Understanding what causes ozone depletion and how certain chemicals thin the ozone layer provides a foundation for improved solutions and technologies. This article explores the causes of ozone depletion, examines hydrofluorocarbons (HFCs) as substitutes for ozone-depleting chemicals, and highlights alternative strategies designed to help restore and protect the ozone layer.
Ozone depletion refers to the gradual reduction of ozone (O₃) in the Earth’s stratosphere. This thinning weakens the planet’s natural shield against ultraviolet radiation. Overexposure to UV can lead to an increase in skin cancers, cataracts in humans, and damage to terrestrial and marine ecosystems. Therefore, understanding how chemicals such as chlorofluorocarbons (CFCs) contribute to this process is essential.
The discovery that synthetic substances can destroy ozone prompted global efforts to identify safer replacements. Many industries initially turned to HFCs, but these substitutes often come with their own environmental drawbacks. Understanding what causes ozone depletion is essential, as technologies advance and scientists continue to develop alternatives that combat ozone depletion without creating other ecological challenges. This article will break down these concepts step by step, providing clear examples of how different substances either help or harm the ozone layer.
What Causes Ozone Depletion?
Definition of Ozone
Ozone is a gas made up of three oxygen atoms (O_3). While it can sometimes form near Earth’s surface and create smog, the vast majority of ozone is found in the stratosphere (10–50 km above Earth). This stratospheric ozone absorbs large amounts of the sun’s UV radiation, protecting organisms from DNA damage and ensuring that life can flourish on land and in water.
Role of the Ozone Layer
Scientists often describe the ozone layer as Earth’s sunscreen. Without it, harmful UV-B radiation penetrates deeper into the planet’s surface. Organisms living on land and in shallow water would face more DNA mutations, which could cause population declines in sensitive species. Moreover, these effects pose risks to our agricultural systems by reducing crop yields.
Main Culprits: CFCs and Other Ozone-Depleting Chemicals
The most notorious ozone-depleting chemicals are chlorofluorocarbons (CFCs). Used for decades in refrigeration, air conditioning, and aerosol sprays, CFCs were once popular due to their chemical stability and low toxicity. However, once CFCs reach the stratosphere, they release chlorine atoms that break apart ozone molecules, illustrating what causes ozone depletion. Other chemicals, such as halons and carbon tetrachloride, display similar properties and also damage the ozone layer.
Example: How CFCs Break Down Ozone Molecules
- CFC molecules travel upward into the stratosphere.
- UV radiation strikes these molecules, causing them to release chlorine (Cl) atoms.
- Each chlorine atom reacts with an ozone molecule (O_3), forming chlorine monoxide (ClO) and an ordinary oxygen molecule (O_2).
- The ClO can combine with a free oxygen atom and release the chlorine atom again.
- This chlorine atom repeats the cycle, destroying more ozone molecules.
This chain reaction continues until the chlorine atom eventually binds with other substances, but at that point, significant ozone depletion may have occurred. Therefore, CFCs present an evident threat to stratospheric ozone.
The Role of Hydrofluorocarbons (HFCs)
What Are HFCs?
Hydrofluorocarbons (HFCs) are synthetic chemicals first introduced as replacements for CFCs. HFCs contain hydrogen, fluorine, and carbon atoms but do not include chlorine or bromine. Because of this difference, HFCs do not directly deplete ozone. This characteristic initially made them attractive alternatives in products like air conditioners and refrigerators.
Why HFCs Were Introduced as Substitutes for CFCs
When the global community realized that CFCs were the primary drivers of ozone depletion, it became necessary to identify safer chemicals. HFCs met that requirement since they lack the chlorine atoms responsible for attacking ozone. Moreover, HFCs often have suitable thermodynamic properties, meaning they worked similarly in refrigeration and cooling systems. Therefore, many industries adopted HFCs as a straightforward fix to the CFC problem.
Pros and Cons of HFCs
- Pros:
- Do not break down ozone molecules.
- Perform effectively in refrigeration and air conditioning.
- Help meet the urgent need to replace CFCs quickly.
- Cons:
- Some HFCs are potent greenhouse gases.
- Contribute to global warming when they escape into the atmosphere.
- May lead to serious climate impacts if used widely without proper containment.
Because HFCs can act as strong greenhouse gases, their increased usage potentially accelerates climate change. Therefore, their uncontrolled release adds to concerns already tied to carbon dioxide (CO_2) emissions.
Example: Comparing CFCs and HFCs
CFCs:
- Ozone depletion potential (ODP): High.
- Global warming potential (GWP): Moderate to high.
- Use: Historically common in older refrigerators and aerosol products.
HFCs:
- Ozone depletion potential (ODP): Zero or near zero.
- Global warming potential (GWP): Can be very high for certain varieties.
- Use: Often used in modern refrigerators, air conditioners, and insulating foams.
In both cases, the impact on Earth’s climate system is critical. Even though HFCs do not damage the ozone layer directly, they can contribute to accelerated global warming.
Alternatives to HFCs and Future Solutions
Overview of Alternative Chemicals
Because some HFCs have a high global warming potential, researchers have explored safer substitutes. These alternatives include:
- Natural refrigerants (like ammonia and hydrocarbons).
- Hydrofluoroolefins (HFOs) that break down more quickly in the atmosphere.
- Advanced systems designed to reduce overall gas leakage.
Innovations in Technology to Combat Ozone Depletion
Reducing or eliminating refrigerants that deplete ozone or contribute heavily to global warming has inspired several technological advancements. Many industries now emphasize leak detection systems to minimize emissions. Additionally, alternative cooling methods—such as magnetocaloric refrigeration—are under investigation to reduce reliance on chemical refrigerants entirely.
Example: How Using Propane as a Refrigerant Can Avoid Ozone Depletion
- Propane (C_3H_8) is a hydrocarbon with no chlorine or bromine atoms.
- Systems that use propane usually have lower global warming potential than many HFC systems.
- A well-optimized propane system has efficient cooling power, reducing energy demand.
- Because propane does not release chlorine, there is no direct risk of ozone molecule destruction.
- Careful handling is required due to its flammable nature, but specialized equipment addresses safety concerns.
Steps Industries Can Take to Minimize Impact
- Transition to low-global-warming-potential refrigerants, such as certain hydrofluoroolefins.
- Enhance product designs to reduce leaks over the appliance’s lifetime.
- Recycle or destroy old refrigerants through proper reclamation programs.
- Invest in service technician training to ensure safe handling, installation, and disposal.
Therefore, industries can avoid creating new environmental problems while solving the old ones. Each step requires cooperation, regulations, and consumer willingness to support more sustainable products.
Ozone Layer Recovery Efforts
International Agreements (e.g., Montreal Protocol)
A significant milestone in protecting the ozone layer is the Montreal Protocol, an international treaty established in 1987. This agreement aimed to phase out the production and use of ozone-depleting substances, including CFCs. Thousands of global stakeholders, from major corporations to local governments, have worked to meet the phaseout schedules set by the treaty.
Success Stories and Current Progress
Under the Montreal Protocol, the manufacture of many dangerous ozone-depleting chemicals has dramatically decreased. As a result, scientists have documented early signs of ozone layer recovery. Measurements indicate reduced chlorine levels in the stratosphere, which suggests that collective efforts are indeed reversing past harm.
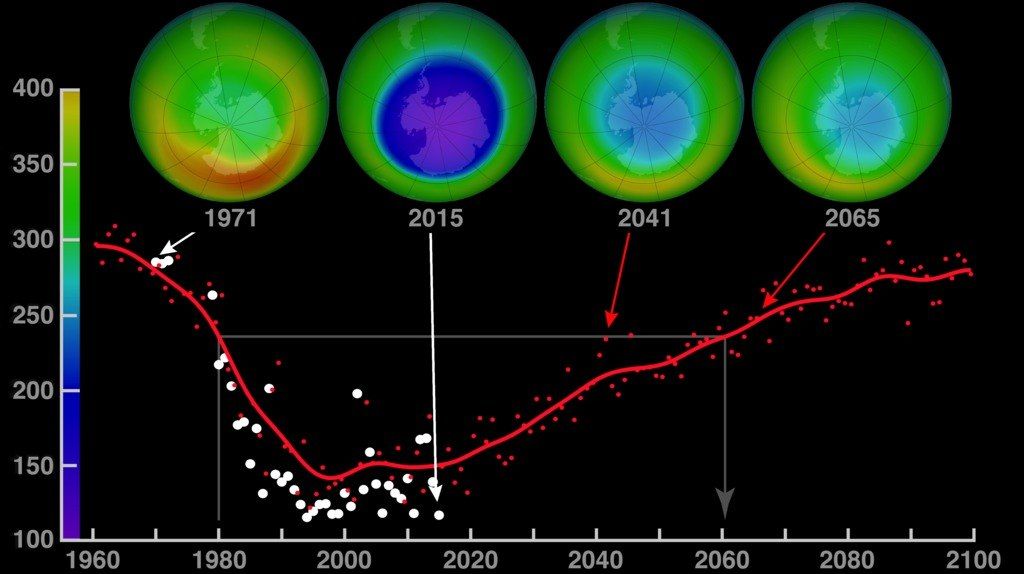
Example: Statistics Showing the Recovery of the Ozone Layer
- The United Nations Environment Programme (UNEP) reports that the ozone layer is on track to return to 1980 levels by around the middle of the 21st century.
- According to some satellite observations, healing is visible in the Antarctic ozone hole.
- Reduction in CFC usage has also led to a measurable reduction in atmospheric chlorine.
Although these trends signal improvement, full restoration may still take decades. Therefore, it remains necessary to maintain strong global cooperation and vigilance in phasing out harmful substances.
Importance of Global Cooperation
Because the atmosphere is shared, actions taken by one country influence the entire planet. When nations collaborate through frameworks like the Montreal Protocol, every region benefits. This agreement highlights that global-scale challenges—whether ozone depletion, greenhouse gas emissions, or biodiversity loss—demand concerted, long-term solutions. Without such collaboration, progress falters, and the environment suffers.
Conclusion
Ozone depletion underscores the interconnectedness of atmospheric processes, industrial practices, and global environmental policies. The widespread use of chlorofluorocarbons nearly led to disastrous consequences for life on Earth. Hydrofluorocarbons offered an initial fix, yet they introduced new complications involving climate change. Now, natural refrigerants and innovative solutions present promising ways to protect the stratospheric ozone layer without intensifying global warming.
Future recovery depends on continued research, practical technological improvements, and enforcement of international agreements. Although significant progress has been made, carefully monitoring ozone-depleting chemicals and encouraging alternatives remain vital. By remaining informed about what causes ozone depletion and supporting environmentally responsible approaches, communities can ensure that the ozone layer continues its healing process. The story of ozone depletion not only reveals how human actions impact the atmosphere, but also demonstrates how concerted global responses can repair and protect crucial planetary systems.
Key Vocabulary
- Ozone (O₃): A gas composed of three oxygen atoms in the stratosphere that protects life by absorbing harmful UV radiation.
- Chlorofluorocarbons (CFCs): Synthetic chemicals once widely used in refrigeration and aerosols, but later found to significantly deplete the ozone layer.
- Hydrofluorocarbons (HFCs): Substitutes for CFCs that do not harm the ozone layer; however, some varieties possess high global warming potential.
- Montreal Protocol: An international treaty established to phase out the production and consumption of ozone-depleting substances.
- Greenhouse Gases: Gases that trap heat in the Earth’s atmosphere, contributing to global warming and climate change.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 8.14 Review
- AP® Environmental Science: 8.15 Review
- AP® Environmental Science: 9.1 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








