What We Review
Introduction
Stratospheric ozone plays a vital role in protecting life on Earth. It is essential to understand how the ozone layer functions, why it is so important, and what causes its depletion. Therefore, this post explains the mechanisms behind stratospheric ozone depletion and highlights solutions taken at the global level to preserve this important protective shield.
Ozone depletion occurs when the concentration of ozone molecules in the stratosphere decreases, allowing more harmful ultraviolet (UV) radiation to reach the Earth’s surface. This phenomenon has attracted global attention because of its potential to harm organisms and disrupt environmental processes. Stratospheric ozone depletion aligns with important learning objectives, such as understanding the evolution of life on Earth and the role of human actions in shaping atmospheric conditions.
Shortly after it was discovered that certain human-made chemicals adversely affect the ozone layer, scientists and policy makers across the globe began collaborating to reverse these effects. Presently, international efforts continue to ensure that future generations inherit an atmosphere with sufficient ozone to protect against excessive UV rays.
What Is the Ozone Layer?
Ozone is a molecule composed of three oxygen atoms, written as O_3. The ozone layer, located primarily in the stratosphere (around 10 to 30 kilometers above the Earth’s surface), forms when ultraviolet radiation breaks apart oxygen molecules (O_2). The free oxygen atoms then bond with intact O_2 molecules to produce ozone:
O_2 + O \rightarrow O_3Because of its high concentration in this layer, ozone effectively absorbs large amounts of UV radiation from the Sun. In simpler terms, the stratospheric ozone layer acts like a protective sunscreen, filtering out harmful wavelengths of light while letting in those that support life processes such as photosynthesis.
Example: Earth’s Sunscreen
- Incoming solar radiation includes harmful UV rays.
- The stratospheric ozone layer intercepts a significant fraction of these harmful rays.
- As a result, surface-level organisms receive less damaging radiation.
This dynamic ensures that life on Earth can thrive without experiencing dangerously high UV exposure levels.
Why Is the Ozone Layer Important?
Without stratospheric ozone, organisms would be exposed to higher levels of UV rays. Although short doses of UV are often manageable, prolonged or intensified exposure can damage DNA, impair plant growth, and reduce plankton populations in oceans. Increased UV radiation also leads to human health problems, including skin cancer and cataracts.
Moreover, changes in the ozone layer can affect ecological interactions. For example, an increase in UV rays may harm phytoplankton, which form the base of many aquatic food webs. Consequently, the ripple effects of ozone depletion could impact entire ecosystems, reflecting the interconnectedness of environmental systems discussed in AP® Environmental Science.
Example: Effects of UV Rays on Living Organisms
- UV rays penetrate through the atmosphere to the Earth’s surface.
- Sensitive cells (in humans, animals, or plants) absorb extra UV energy.
- DNA and other cell components may become damaged.
- Organisms experience negative health effects, such as increased cancer risk or inhibited growth.
Therefore, preventing further ozone depletion helps maintain a healthy balance for species as well as entire ecosystems.
Causes of Stratospheric Ozone Depletion
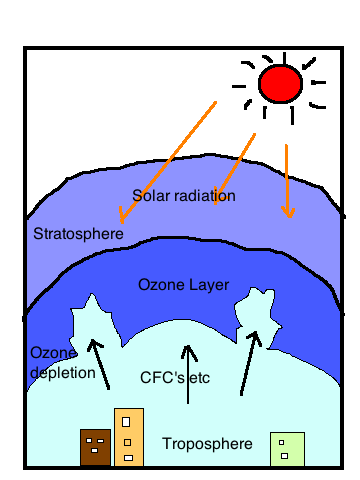
Human (anthropogenic) and natural factors contribute to the thinning of the ozone layer. While science shows that humans have dramatically accelerated ozone depletion through certain chemicals, nature also plays a part. However, the most significant driver of long-term ozone loss is linked to human-produced chlorofluorocarbons (CFCs).
Chlorofluorocarbons (CFCs)
Chlorofluorocarbons are synthetic chemicals once widely used in refrigerants, aerosol propellants, and foam-blowing agents. They were favored for their stability and non-toxicity at ground level. However, their stability also allows them to drift into the stratosphere, where they then break apart under intense solar radiation. As the CFC molecules split, they release chlorine atoms that attack ozone molecules repeatedly:
- Cl atoms separate from CFCs in the stratosphere.
- A free Cl atom reacts with an O_3 molecule: Cl + O_3 \rightarrow ClO + O_2
- The newly formed chlorine monoxide (ClO) can later release the same chlorine atom, which continues to break down additional ozone molecules.
Therefore, one chlorine atom can destroy thousands of O_3 molecules before finally inactivating through other reactions. This process illustrates why even small amounts of CFC emissions can contribute to significant ozone loss over time.
Natural Factors
In addition to human activity, natural processes also influence ozone concentrations. For instance, the melting of ice crystals within polar stratospheric clouds can speed up ozone destruction, particularly in the Antarctic spring. During the polar winter, ice crystals trap compounds like ClO; when spring sunlight arrives, these previously inactive compounds are released, triggering rapid ozone reduction. Hence, seasonal variations in polar regions lead to the well-known “ozone hole” that forms over Antarctica each year.
Consequences of Ozone Depletion
Stratospheric ozone depletion leads to more UV rays reaching the Earth’s surface. Therefore, the risk of harmful effects on humans and other organisms increases significantly.
Health Effects: Skin Cancer and Cataracts
UV radiation can penetrate skin cells, causing mutations that may result in skin cancer. Additionally, too much UV exposure raises the likelihood of cataracts, a condition where the lens of the eye becomes cloudy. Because these health concerns are often linked to the cumulative effect of UV exposure, long-term ozone thinning amplifies the danger for people spending extended hours outdoors.
Environmental Effects: Impact on Ecosystems
Environmentally, increased UV levels stress marine systems by harming plankton in the upper layers of ocean water. Since plankton serve as a key food source for many marine organisms, a domino effect can occur throughout the food chain. Terrestrial plants can also suffer from impaired growth and reduced yield, thereby influencing entire habitats. Consequently, ozone depletion poses a threat to biodiversity, ecosystem productivity, and agricultural systems.
Example: Chain Reaction from Ozone Depletion to Skin Cancer
- CFCs are emitted and travel to the stratosphere.
- Chlorine atoms are released, reducing the amount of O_3 in the stratosphere.
- Lower O_3 concentrations allow more UV radiation to pass through.
- Humans exposed to more UV radiation face elevated risks of skin mutations, raising cancer rates.
Solutions and Global Efforts
Scientists recognized the threat posed by ozone depletion decades ago, prompting collaborative solutions. International treaties, government regulations, and industry innovations have all aimed to address the crisis. Therefore, cooperation between nations became essential in successfully limiting ozone-harming substances.
The Montreal Protocol
One of the most significant steps is the Montreal Protocol, an international treaty signed in 1987. It mandated the gradual phase-out of ozone-depleting substances such as CFCs. Over time, participating countries have reduced the production and consumption of these chemicals, shifting to more ozone-friendly alternatives. According to data collected over multiple decades, the ozone layer shows signs of recovery, demonstrating the effectiveness of this coordinated global response.
Ongoing Challenges
Although the Montreal Protocol is widely hailed as a success, there are still issues to overcome. For example, some replacement chemicals also have high global warming potential, raising concerns related to climate change. Additionally, illegal production of banned substances sometimes goes undetected. Therefore, consistent monitoring and enforcement are vital to ensure the maximum benefit for the ozone layer, climate health, and global communities.
Example: Tracking Ozone Recovery
- Satellite observations measure ozone concentrations over the poles.
- Scientists compare current readings to historical data.
- Gradual increases in ozone levels indicate partial restoration.
- Continued cooperation ensures further improvements.
These measurements, along with ground-based monitoring, provide a window into how policy decisions and scientific efforts influence atmospheric health.
Conclusion
Understanding stratospheric ozone depletion offers insight into how human actions can reshape the environment at a global scale. The ozone layer’s restoration involves the combined efforts of scientists, governments, and industries dedicated to phasing out harmful substances. Many students analyze the implications of policy strategies like the Montreal Protocol and observe how science-based legislation can create tangible progress.
Nevertheless, sustained education and awareness remain essential. Monitoring ozone trends, evaluating less harmful chemical alternatives, and furthering public knowledge protect not only the ozone layer but also comprehensively address broader environmental concerns, such as the carbon cycle and climate change. By exploring these interconnected processes, students gain a deeper appreciation for how environmental policies can secure healthier ecosystems and societies worldwide.
Key Vocabulary
- Ozone Layer: A region of Earth’s stratosphere rich in O_3 molecules, which shields life from the Sun’s harmful ultraviolet radiation.
- Chlorofluorocarbons (CFCs): Stable synthetic compounds once used in refrigerants and aerosols that break down ozone molecules in the stratosphere.
- UV Rays: Invisible electromagnetic waves that can damage living tissues; higher exposure results from reduced stratospheric ozone.
- Cataracts: A condition where the lens of the eye becomes cloudy, often linked to excessive exposure to UV radiation.
- Montreal Protocol: A landmark international treaty signed in 1987 to phase out the production and consumption of ozone-depleting substances.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 8.13 Review
- AP® Environmental Science: 8.14 Review
- AP® Environmental Science: 8.15 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








