What We Review
Introduction
Climate science aims to understand how different elements in Earth’s atmosphere and surface influence global temperatures. Among the most critical processes is the greenhouse effect, which helps maintain a temperature suitable for life. When sunlight reaches Earth, some energy is absorbed by land and water surfaces, while some is reflected back into space. However, an important fraction of that heat then radiates upward as infrared energy., which describes the greenhouse effect as certain gases in the atmosphere trap part of this heat, re-radiating it toward the surface and the lower atmosphere.
The greenhouse effect can be defined simply as the process by which certain gases in Earth’s atmosphere hold in heat. Without these gases, global temperatures would be far lower and life as it is known would not be possible. Therefore, understanding how these gases function is vital for anyone interested in climate science.
Understanding the Greenhouse Effect
The greenhouse effect begins with solar radiation passing through Earth’s atmosphere. Sunlight warms various surfaces, which then emit infrared radiation (heat). Greenhouse gases absorb and re-radiate portions of this infrared energy, creating an insulating effect.
Consequently, the atmosphere retains heat that would otherwise escape into space. This natural mechanism keeps the planet at an average temperature that supports thriving ecosystems. However, excess greenhouse gases increase this warming effect and can disrupt Earth’s climate balance.
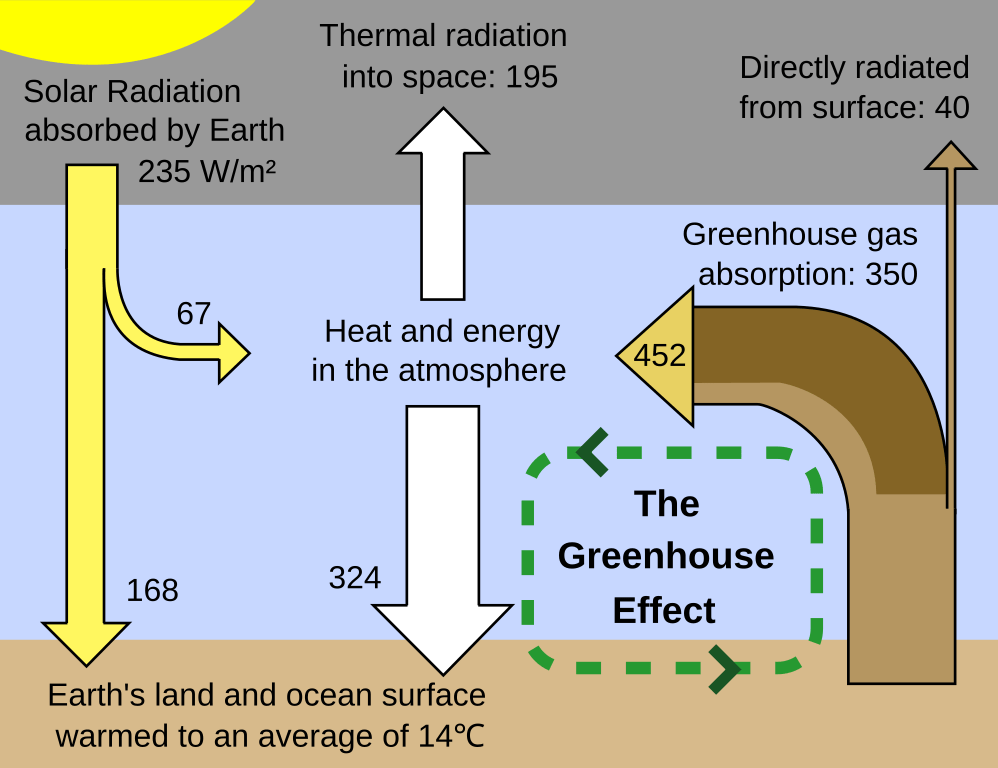
A “Glass House” Analogy
Visualizing a greenhouse can clarify how the greenhouse effect works:
- Sunlight enters the greenhouse through the glass.
- Plants and soil absorb the sunlight and convert much of it to heat.
- The heat attempts to escape, but the glass walls trap most of the outgoing infrared radiation.
- As a result, the interior remains warmer than the outside air.
In Earth’s case, greenhouse gases play a role similar to the glass walls. They let in sunlight but trap a significant portion of the heat heading back into space.
Principal Greenhouse Gases
Greenhouse gases come in various forms. Each one has different origins and contributes to climate change in distinct ways. The principal greenhouse gases include carbon dioxide (CO2), methane (CH4), water vapor (H2O), nitrous oxide (N2O), and chlorofluorocarbons (CFCs).
- Carbon Dioxide (CO2)
- Carbon dioxide is produced by activities like burning fossil fuels (coal, oil, and natural gas) and deforestation. It is often considered the most important greenhouse gas due to its abundance and lengthy residence time in the atmosphere.
- Methane (CH4)
- Methane arises from sources such as livestock digestion, landfills, and the production of natural gas. Although it stays in the atmosphere for a shorter duration than CO2, it is more potent in trapping heat per molecule.
- Water Vapor (H2O)
- Water vapor is the most abundant greenhouse gas in terms of volume. Yet, it does not stay in the atmosphere for very long. Consequently, it is not considered a major driver of long-term climate change.
- Nitrous Oxide (N2O)
- Nitrous oxide originates from agricultural practices like fertilizer application and from industrial processes. It can remain in the atmosphere for over a century, further intensifying its impact on global temperatures.
- Chlorofluorocarbons (CFCs)
- CFCs are human-made chemicals formerly used in refrigerants and aerosols. They have been mostly phased out due to their destructive effect on the ozone layer. Nevertheless, they are still potent greenhouse gases when they do reach the atmosphere.
Example: Sources of Carbon Dioxide in Daily Life
Many routine activities emit CO2 without drawing much attention. For instance, driving a gasoline-powered car to school produces CO2 through fuel combustion. Similarly, using electricity generated from coal or natural gas contributes to CO2 output. Over time, these daily emissions accumulate and drive the rising concentration of carbon dioxide in the atmosphere.
Global Warming Potential (GWP)
Global Warming Potential (GWP) is a metric that compares the warming influence of different greenhouse gases over a specific period, usually 100 years. Carbon dioxide has a GWP of 1 and serves as the reference for comparing other gases.
Other greenhouse gases have much higher GWPs:
- Methane’s GWP is approximately 25–28 times that of CO2.
- Nitrous oxide’s GWP exceeds 250 times that of CO2.
- CFCs can have GWPs in the thousands or tens of thousands.
Why GWP Matters
Measuring GWP helps policy makers and scientists prioritize which emissions should be reduced most urgently. Even small amounts of a high-GWP gas can cause significant warming compared to a larger quantity of CO2.
Example: Calculating Methane’s Warming Influence
Suppose 5 kilograms of methane leak into the atmosphere from a landfill. Methane’s GWP is about 25 times greater than CO2. The effective warming potential (\text{Impact}) can be estimated as: \text{Impact} = \text{Mass of CH4} \times \text{GWP of CH4}
Thus, \text{Impact} = 5 , \text{kg} \times 25 = 125 , \text{CO2-equivalent kg}
Therefore, these 5 kilograms of methane would contribute as much warming as roughly 125 kilograms of CO2 over the chosen time frame.
The Role of Water Vapor
While water vapor is indeed a greenhouse gas, it has a short residence time in the atmosphere. In fact, it usually stays in gaseous form for only a matter of days, cycling rapidly through evaporation and precipitation processes. Therefore, water vapor varies widely based on regional conditions and is not the key long-term driver of global warming.
Example: Water Vapor and Daily Weather
On rainy days, higher moisture levels can briefly increase local humidity, causing more heat to remain near the surface. However, once the rain stops, water vapor either condenses or disperses quickly. This short cycle contrasts with gases such as CO2 and N2O, which remain in the atmosphere for decades or centuries.
Impact of the Greenhouse Effect on Climate Change
The enhanced greenhouse effect, driven by excessive amounts of greenhouse gases, influences global temperatures. More trapped heat leads to higher average temperatures, shifting precipitation patterns, and altering weather extremes. Over time, these changes can disrupt ecosystems and resources like freshwater supply.
Industrialization has accelerated these processes considerably. Historically, concentrations of CO2 in the atmosphere began rising with the widespread use of coal and later, oil and gas. Consequently, global temperatures have shown an upward trend, aligning with increases in greenhouse gas emissions.
Example: Rising CO2 Levels Since the Industrial Revolution
Scientists often connect the beginning of the Industrial Revolution, around the late 18th century, to a gradual rise in atmospheric CO2. Monitoring stations around the world record the building concentrations of CO2, which now exceed 400 parts per million—significantly higher than pre-industrial levels. This correlation suggests a link between industrial activity and global warming.
Mitigating Climate Change
Reducing the impact of the greenhouse effect involves lowering emissions from key sources. Governments, industries, and individuals can all play a role in transitioning toward more sustainable practices.
- Switch to Renewable Energy
- Solar, wind, and hydroelectric power produce fewer greenhouse gases during operation. Using these sources helps replace carbon-intensive coal- or gas-fired power plants.
- Carbon Capture and Storage (CCS)
- This technology captures CO2 emissions from industrial sites before they reach the atmosphere. The captured CO2 is stored underground in rock formations to prevent it from contributing to climate change.
- Improve Energy Efficiency
- Upgrading insulation in buildings, optimizing vehicle fuel efficiency, and using energy-efficient appliances reduce overall energy demand. Consequently, fewer emissions are generated.
- Green Transportation
- Public transit, electric vehicles, and carpooling can help reduce the number of gasoline-powered engines generating CO2 on the roads.
- Sustainable Agriculture
- Adjusting fertilizer methods and managing livestock operations more carefully can cut emissions of nitrous oxide and methane, respectively.
- Waste Reduction
- Methane arises from landfills, where organic waste decomposes. However, recycling and composting programs reduce the amount of waste that becomes methane.
Conclusion
In summary, the greenhouse effect is a crucial natural process that warms our planet and allows life to flourish. Nevertheless, rising concentrations of greenhouse gases have greatly amplified this effect, leading to global warming and climate instability. Being familiar with the principal greenhouse gases—CO2, CH4, H2O, N2O, and CFCs—and their respective Global Warming Potentials is an essential step in understanding how human actions influence climate systems. More importantly, mitigating these effects depends on concerted efforts at every level, from developing cleaner energy sources to practicing sustainable lifestyles.
Exploring additional information on greenhouse gas emissions, carbon cycles, and climate solutions can further enrich understanding. Ultimately, knowledge about the greenhouse effect and its impact equips students with the perspectives needed to engage in environmental decision-making and action.
IX. Key Vocabulary
- Greenhouse Effect: The trapping of heat in Earth’s atmosphere by certain gases that allows life-sustaining temperatures.
- Global Warming Potential (GWP): A measure of how much heat a greenhouse gas traps relative to carbon dioxide.
- Carbon Dioxide (CO2): A common greenhouse gas released primarily through fossil fuel combustion and deforestation.
- Methane (CH4): A potent greenhouse gas produced by sources like livestock and landfills, with a high GWP.
- Chlorofluorocarbons (CFCs): Human-made chemicals with very high GWP, now largely banned due to ozone layer damage.
Sharpen Your Skills for AP® Environmental Science
Are you preparing for the AP® Environmental Science test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world AP® Environmental Science problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
- AP® Environmental Science: 8.15 Review
- AP® Environmental Science: 9.1 Review
- AP® Environmental Science: 9.2 Review
Need help preparing for your AP® Environmental Science exam?
Albert has hundreds of AP® Environmental Science practice questions, free response, and full-length practice tests to try out.








